Figures & data
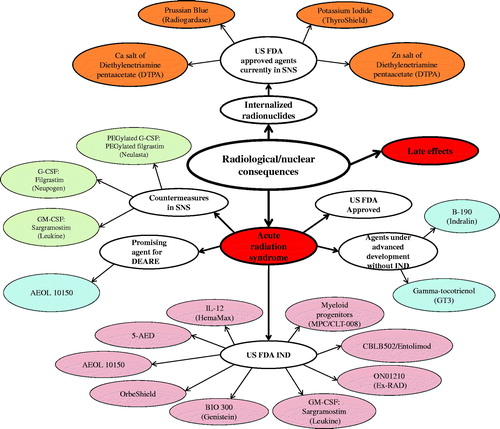
Figure 1. Current status of radiation countermeasures for radiological and nuclear threats. Three countermeasures for ARS (Neupogen, Neulasta, and Leukine) and four agents for internalized radionuclides (Prussian Blue, Potassium iodide, Ca-DTPA, and Zn-DTPA) have been procured for SNS, and nine radiation countermeasures have U.S. FDA IND. Color coding: red, acute and late-arising injuries; light pink, MCM with FDA IND status; baby blue, new countermeasures under development without IND status; burnt orange, FDA fully-approved medicals for internalized radionuclides; light green, FDA fully-approved medicals for ARS within the SNS.