Figures & data
Figure 1. Palmitoylation and subcellular trafficking. (a) Golgi-targeted zDHHC PATs mediate the palmitoylation of substrates for substrate stabilization at Golgi membranes prior to their packaging into vesicles and forward-trafficking to the plasma membrane. (b) Three main zDHHC PATs, zDHHC2, zDHHC5 and zDHHC8, are active PATs targeted to the plasma membrane (PM), while zDHHC6 is an active PAT present on ER membranes. These non-Golgi PATs are appropriately positioned to palmitoylate substrates locally, and govern substrate targeting to these same membranes. The precise localization of non-Golgi PATs is critical for the proper non-Golgi-membrane localization of their substrates following palmitoylation, and may be an important predictor of PAT-substrate specificity. Created with BioRender.com.
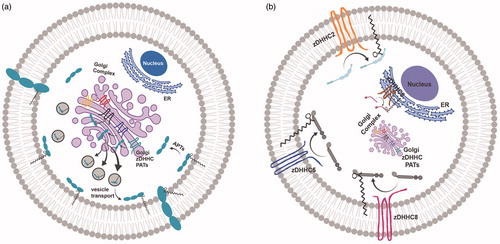
Figure 2. Neuronal activity-regulated translocation of zDHHC5 for local palmitoylation of its synaptic substrates. (1a) zDHHC5 cycles from the dendritic shaft to dendritic spines on recycling endosomes (REs) to palmitoylate GRIP1b. (1b) Palmitoylation of GRIP1b by RE-localized zDHHC5 targets GRIP1b to REs to enable GRIP1b binding to KIF5, which mediates the proper trafficking and stabilization of AMPAR GluA2 subunits to the PM (1c). (2a) Under basal conditions, zDHHC5 is stabilized at the postsynaptic membrane through Fyn-mediated phosphorylation, which prevents its association with endocytic machinery. (2b) Upon increase in neuronal activity, zDHHC5 translocates from the postsynaptic membrane to dendritic shafts on REs to recruit its substrates. (2c) Once arrived at the shaft, zDHHC5 palmitoylates its substrate δ-catenin. (2d) Palmitoylated δ-catenin is trafficked to the postsynaptic membrane on REs with zDHHC5. (2e) Palmitoylation-dependent δ-catenin recruitment at post-synaptic membranes binds and stabilizes N-cadherin, leading to enhanced synaptic plasticity. Created with BioRender.com.
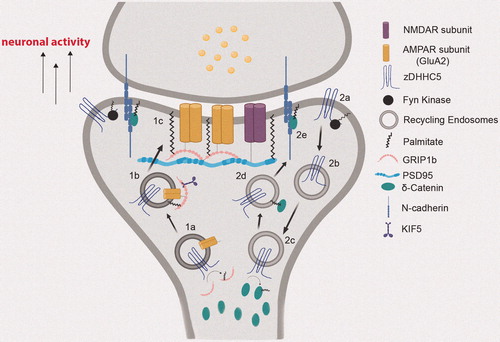
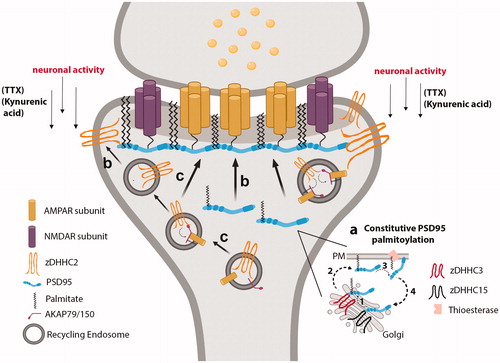
Figure 3. RE- and PSD-localized zDHHC2 locally palmitoylates and targets synaptic substrates to REs and the PSD. (a) PSD95 is constitutively palmitoylated by Golgi-localized zDHHC3 and zDHHC15, which promotes its forward trafficking to the PM. A thioesterase depalmitoylates PSD95 to drive its retrograde trafficking back to the Golgi. (b) Under conditions of decreased neuronal activity, REs containing zDHHC2 deliver zDHHC2 to the PSD. PSD-localized zDHHC2 then interacts with PSD95 to palmitoylate and target PSD95 to the PSD. (c) RE-localized zDHHC2 mediates the palmitoylation of AKAP79/150, to promote the proper delivery of AMPARs at the post-synaptic membrane during LTP. Created with BioRender.com.