Figures & data
Figure 1. Proton decoupled phosphorous-31 NMR spectra of DoMPC/DoHPC bicelles (q = 3, 20% w/v, 35°C) (A) and with the M4-TMD incorporated at a lipid to protein ratio of 50:1 (B).
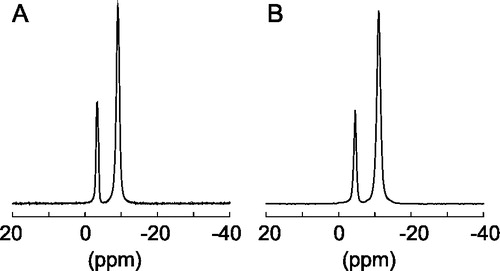
Figure 2. Variation of phosphorous-31 chemical shift as a function of temperature for both DoMPC/DoHPC and DoMPC/DoHPC/M4 bicelles. Phosphorous-31 chemical shifts for bicelles in the absence and presence of M4 are indicated. In absence of M4, DoMPC (♦) and DoHPC(⋄) and in the presence of M4 DoMPC(•) and DoHPC(○).
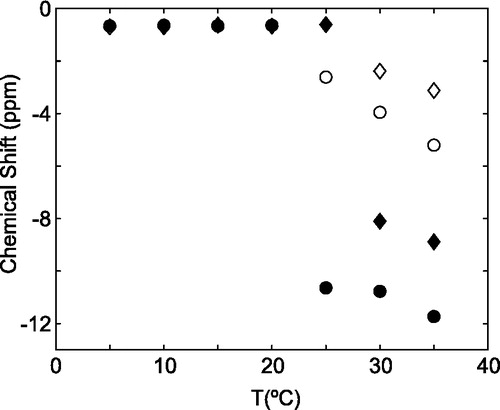
Figure 3. CD spectra of the M4-TMD dissolved in TFE (solid line) and reconstituted bicelles (dotted line). Both spectra recorded at 35°C.
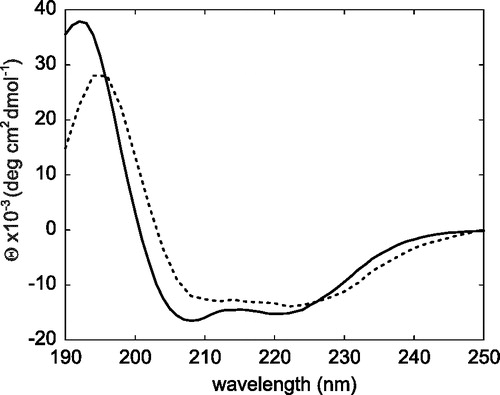
Table I. Characterization of the anisotropic interactions present at the labelled sites within the lyophilised M4-TMD.
Figure 4. Spectra showing the effect of the lipid phase on the dynamics observed by the M4-TMD. Spectra of the M4-TMD reconstituted into DoMPC vesicles below (5°C) (A–C) and above (35°) (D–F) the gel to liquid crystalline phase transition temperature. Proton decoupled 13C spectrum, with magic-angle spinning, ωr=1.86kHz (A) and static (D). Cross polarization 15N spectra ωr=1.735 kHz at 5°C (B) and ωr=1.700 at 35°C (E). Deuterium quadrupolar echo lineshapes at 5°C (C) and 35°C (F).
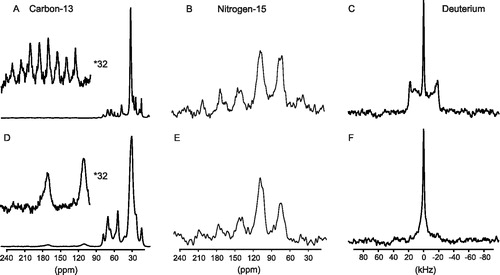
Figure 5. Spectra showing effect of macroscopic orientation of bicelles on the solid state NMR spectra of the M4-TMD. Isotropic spectra (A–C) and macroscopically aligned (D–F). 13C and 15N isotropic spectra acquired with magic angle spinning, 2H isotropic spectra acquired below the phase transition of the bicelles (5°C). Oriented lineshapes acquired on static samples at 35°C (asterisks indicate the resonances arising from the carbon-13 labelled sites within the peptide).
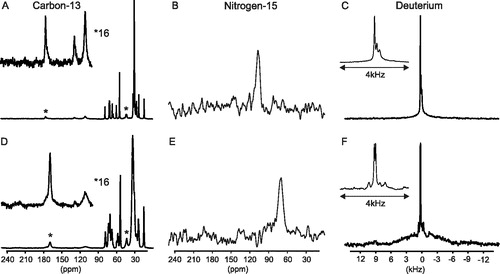
Figure 6. The relationship between the anisotropic interaction present in the M4-TMD and how they are related to the morphology of the bilayer, together with the motional processes that can lead to the averaging of the anisotropic interactions. The initial transformation (ΩPM) transforms the PAS to the molecular frame (MF), in this case defined as collinear with the helix long axis. The second transformation (ΩMB) determines the orientation of the peptide with respect to the bilayer normal (bicelle frame, BF). In the case of vesicles the bicellar frame is defined as collinear with the director frame (DF). For bicelles, fluctuations between the bicelle normal and the director are accounted for by the transformation (ΩMB) and gives rise to the order parameter Sbic. The final transformation between the director and the lab frame (ΩDL) is characterized by the order in the sample. In the case of vesicles ΩDL is characterized by a powder distribution. In bicelles ΩDL is characterized by a Lorentzian distribution about the director normal. This figure is reproduced in colour in Molecular Membrane Biology online.
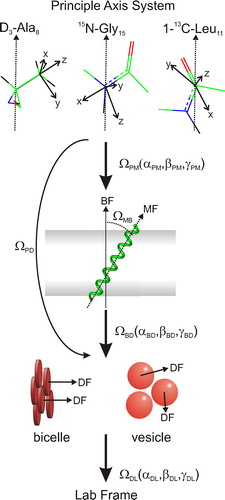
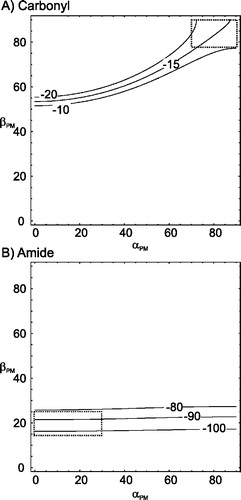
Figure 7. Contour plots showing how the chemical shielding anisotropy for the carbonyl (A) and amide (B) tensor averaged as a function of the Euler angles α and β which relate them to the axis of motional averaging. The contours plotted indicate the values for the averaged chemical shielding anisotropy (in ppm) which are consistent with the experimentally determined values for the carbonyl (A) and amide (B) tensors. Additional contours show the expected deviation in Euler angles upon varying the observed chemical shielding anisotropy by ±5 ppm (A) and ±10 ppm (B). The values plotted are calculated according to equation 1 assuming a static carbonyl tensor with an anisotropy of −85 ppm and asymmetry of 0.65 (A) and a amide tensor of −90 ppm and asymmetry of 0.17 (B). The areas highlighted indicate the areas where the Euler angles would relate the PAS of the chemical shielding anisotropy to the axis of motional averaging consistent with an α-helix.