Figures & data
Figure 1. Schematic of PrPC. Nascent murine PrPC is a protein of 254 amino acids. On translocation into the ER the N-terminal signal peptide (chequered box; red online) and the C-terminal GPI anchor addition signal (wavy line box; green online) are removed and the latter replaced with a GPI anchor. The polybasic region (black; blue online; residues 23–28) at the N-terminus of mature PrPC, the copper-binding octapeptide repeats (stippled; purple online; residues 51-91), the central hydrophobic, neurotoxic domain (diagonal lines; light blue online; residues 106-126) and the two N-linked glycans (black lollipops; Asn-180 and Asn-196) are indicated. The positions of the raft targeting domain, the basolateral targeting domain and the sphingolipid binding domain (see text for details) are indicated below the protein. This figure is reproduced in colour in Molecular Membrane Biology online.
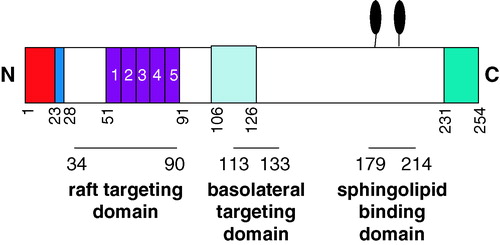
Figure 2. PrPC is localized in rafts but translocates out of them before being endocytosed through clathrin-coated pits. PrPC is attached to the exoplasmic leaflet of the plasma membrane via its GPI anchor and localises within detergent-insoluble rafts through interactions between its N-terminal region (residues 23-90) and a raft-resident protein (grey; orange online) or lipid. Upon Cu2 + binding to the octapeptide repeats the protein undergoes a conformational change that dissociates it from the raft-resident partner and PrPC then moves laterally out of the rafts into detergent-soluble regions of the plasma membrane. The polybasic N-terminal region (blue online) then interacts with the ectodomain of a transmembrane protein (grey; turquoise online) that engages, via its cytoplasmic domain, with the adaptor protein AP-2 and the endocytic machinery of clathrin-coated pits. Modified from Citation[56]. This figure is reproduced in colour in Molecular Membrane Biology online.
![Figure 2. PrPC is localized in rafts but translocates out of them before being endocytosed through clathrin-coated pits. PrPC is attached to the exoplasmic leaflet of the plasma membrane via its GPI anchor and localises within detergent-insoluble rafts through interactions between its N-terminal region (residues 23-90) and a raft-resident protein (grey; orange online) or lipid. Upon Cu2 + binding to the octapeptide repeats the protein undergoes a conformational change that dissociates it from the raft-resident partner and PrPC then moves laterally out of the rafts into detergent-soluble regions of the plasma membrane. The polybasic N-terminal region (blue online) then interacts with the ectodomain of a transmembrane protein (grey; turquoise online) that engages, via its cytoplasmic domain, with the adaptor protein AP-2 and the endocytic machinery of clathrin-coated pits. Modified from Citation[56]. This figure is reproduced in colour in Molecular Membrane Biology online.](/cms/asset/41059f3f-43a3-424c-9aa9-9f68630d5585/imbc_a_144982_f0002_b.jpg)
Figure 3. A model for the role of lipid rafts in the conversion of PrPC to PrPSc and subsequent disease progression. PrPC is attached to the membrane via its GPI anchor and upon clustering in lipid rafts transduces neuroprotective signals into the cell. Infectious PrPSc inserts into the target cell membrane alongside the PrPC in the rafts. Conversion of the PrPC to PrPSc may affect signalling events involving PrPC, leading to neurotoxicity and cell death. Reproduced with permission from Citation[91]. This figure is reproduced in colour in Molecular Membrane Biology online.
![Figure 3. A model for the role of lipid rafts in the conversion of PrPC to PrPSc and subsequent disease progression. PrPC is attached to the membrane via its GPI anchor and upon clustering in lipid rafts transduces neuroprotective signals into the cell. Infectious PrPSc inserts into the target cell membrane alongside the PrPC in the rafts. Conversion of the PrPC to PrPSc may affect signalling events involving PrPC, leading to neurotoxicity and cell death. Reproduced with permission from Citation[91]. This figure is reproduced in colour in Molecular Membrane Biology online.](/cms/asset/20aff8b2-4bdf-4aad-855f-5012f8fc3fbe/imbc_a_144982_f0003_b.jpg)