Figures & data
Figure 1. Asexual erythrocytic lifecycle of Plasmodium falciparum. Human malarial infection commences when an infected Anopheline mosquito bites a susceptible person. Transmitted sporozoites (not shown) spend the first 5–20 days replicating in the liver, without clinical symptoms. Liver-stage infection culminates with the production of thousands of merozoites that enter the bloodstream, where they rapidly adhere to and invade mature erythrocytes. The apical organelles (rhoptries shown) are involved in invasion and vacuole formation. Once inside, the intraerythrocytic lifecycle of Plasmodium falciparum is 48 hours long. Throughout this period, the growing parasite resides in a membranous sack, called the parasitophorous vacuolar membrane (PVM). Immediately following invasion, merozoites change into ring-shaped parasites during the first 24 hours of the lifecycle. From 24–36 hours post-invasion, trophozoite-stage parasites replicate their DNA and organelles and develop a tubovesicular transport network (TVN), which is used to move nutrients and wastes in and out of the cell. During the last 12 hours of the cycle, mature schizont-stage parasites segment into 16–32 daughter merozoites, rupture from the cell and subsequently infect additional erythrocytes. N = nucleus. This Figure is reproduced in color in Molecular Membrane Biology online.
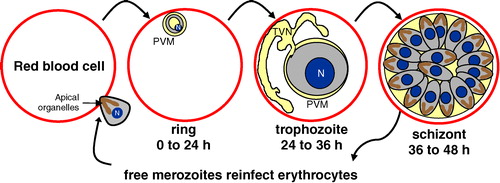
Figure 2. Selective uptake of a subset of erythrocyte DRM proteins into the PVM. Immunofluorescence assays demonstrated that some DRM proteins (i.e., flotillin-1) are strongly recruited to the vacuolar membrane, whereas other DRM proteins (i.e., stomatin/band 7) are excluded from the PVM. The integral membrane protein, band 3, which is a major constituent of erythrocyte DRMs, does not enter the PVM. Further, non-DRM proteins do not traffic to the PVM (i.e., CD47). Cumulatively, the data suggests that malarial invasion and vacuole formation involves an active mechanism to sort host raft proteins that enter the vacuolar membrane. The nuclei were visualized by Hoechst DNA staining. Diameter of erythrocytes ∼7 µm. Adapted from Murphy and Samuel Citation[11] with permission from Blood. This Figure is reproduced in color in Molecular Membrane Biology online.
![Figure 2. Selective uptake of a subset of erythrocyte DRM proteins into the PVM. Immunofluorescence assays demonstrated that some DRM proteins (i.e., flotillin-1) are strongly recruited to the vacuolar membrane, whereas other DRM proteins (i.e., stomatin/band 7) are excluded from the PVM. The integral membrane protein, band 3, which is a major constituent of erythrocyte DRMs, does not enter the PVM. Further, non-DRM proteins do not traffic to the PVM (i.e., CD47). Cumulatively, the data suggests that malarial invasion and vacuole formation involves an active mechanism to sort host raft proteins that enter the vacuolar membrane. The nuclei were visualized by Hoechst DNA staining. Diameter of erythrocytes ∼7 µm. Adapted from Murphy and Samuel Citation[11] with permission from Blood. This Figure is reproduced in color in Molecular Membrane Biology online.](/cms/asset/d4102eca-c1fb-4104-b13e-04036b0321ef/imbc_a_147327_f0002_b.jpg)
Figure 3. Model of erythrocyte DRM rafts and their enrichment in the malarial vacuolar membrane. The uninfected erythrocyte membrane contains a variety of generalized lipid domains (grey spheres) and raft microdomains (pink spheres) containing various proteins. Some proteins partition mostly into DRM raft domains (i.e., flotillins), while others are only minimally present there (i.e., band 3). During malaria infection, merozoite-stage parasites invade erythrocytes to reside in a membrane-bound parasitophorous vacuole. The PVM becomes selectively cholesterol-enriched, and ten of the known raft proteins are internalized to the PVM (flotillin-1 and -2, Gs, β2AR, AQP1, Duffy, CD55, CD58, CD59, scramblase). Major integral membrane proteins are not internalized to the PVM (i.e., glycophorins A and C, cytoskeleton-associated band 3, etc.). The lower left inset shows the perspective of the model, depicting a single infected erythrocyte with a magnified view of the plasma membrane and PVM. Since the PVM is formed by invagination of the plasma membrane, proteins that are cytoplasmically-oriented in uninfected cells remain so upon infection; protein structures exposed to the extracellular space face the vacuolar space upon infection. 4.1 indicates protein 4.1.
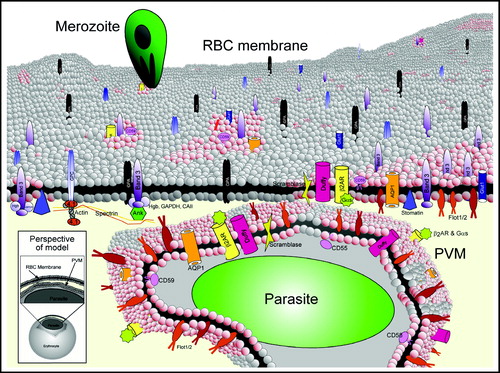
Table I. Summary of erythrocyte lipid raft proteins.
Figure 4. Proposed model for recruitment of parasite and host DRM rafts during PVM formation. The PVM contains proteins originating from erythrocyte and parasite DRMs. The model shows a nascent vacuole (blue circle) where proteins from parasite DRM rafts (colored dots) nucleate host DRM rafts that contain Gs (blue bar). These host-parasite complexes are sequestered in the PVM, where they are located on the cytoplasmic face of the vacuolar membrane. These complexes may be involved in the raft-induced invagination of the host plasma membrane required for PVM formation. PPM indicates parasite plasma membrane. Reproduced from Hiller and colleagues Citation[52].
![Figure 4. Proposed model for recruitment of parasite and host DRM rafts during PVM formation. The PVM contains proteins originating from erythrocyte and parasite DRMs. The model shows a nascent vacuole (blue circle) where proteins from parasite DRM rafts (colored dots) nucleate host DRM rafts that contain Gs (blue bar). These host-parasite complexes are sequestered in the PVM, where they are located on the cytoplasmic face of the vacuolar membrane. These complexes may be involved in the raft-induced invagination of the host plasma membrane required for PVM formation. PPM indicates parasite plasma membrane. Reproduced from Hiller and colleagues Citation[52].](/cms/asset/4f004a00-17c9-48dd-ae0d-7eab81512752/imbc_a_147327_f0004_b.jpg)