Figures & data
Figure 1. Effects of Na+ and H+ on the transport activities of recombinant hCNT1, hCNT2 and hCNT3 produced in Xenopus oocytes. Radiolabelled fluxes of uridine (20 µM, 20°C, 1 min flux) in oocytes injected with RNA transcripts encoding hCNT1 (A), hCNT2 (B), or hCNT3 (C) were measured in transport media containing Na+ (open bar; 100 mM NaCl, pH 8.5) or choline (gray bars; 100 mM ChCl, pH 5.5–8.5). Values were corrected for basal non-mediated uptake in control water-injected oocytes and are means±SEM of 10–12 oocytes. The experiment was performed on a single batch of oocytes used on the same day. Representative cation/nucleoside current traces in single hCNT1- (D), hCNT2- (E), or hCNT3- (F) producing oocytes clamped at −50 mV in transport medium containing Na+ (100 mM NaCl, pH 8.5) or choline (100 mM ChCl, pH 5.5). The bar denotes addition of uridine (100 µM) to the bath. No currents were observed in control water-injected oocytes (traces not shown).
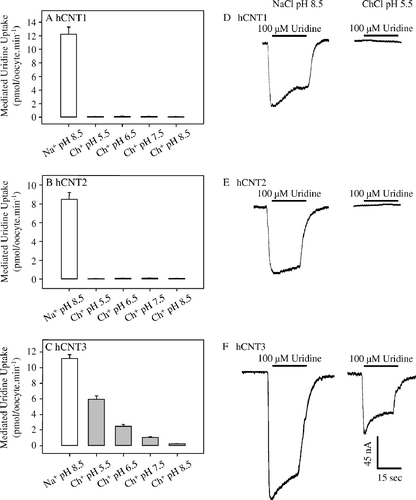
Figure 2. Cation activation kinetics of hCNT1, hCNT2 and hCNT3. Initial rates of 14C-uridine uptake (20 µM, 20°C, 1 min flux) were measured in Na+-containing (0–100 mM NaCl) transport media at pH 8.5 in oocytes injected either with water alone (open circles) or with water containing RNA transcripts encoding hCNT1 (A), hCNT2 (B) or hCNT3 (C) (solid circles). All of the fluxes were performed on the same batch of oocytes used on the same day. Kinetic parameters derived from these data for the hCNT-mediated component of transport (uptake in RNA transcript-injected oocytes minus uptake in water-injected oocytes) are presented in . The inset in each graph shows the Hill plot for the data.
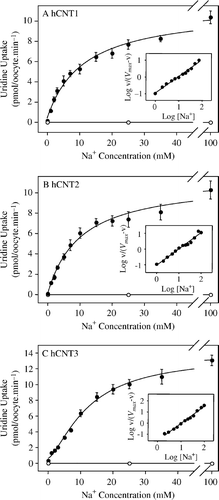
Table I. Na+ activation kinetics of uridine uptake by hCNT1, hCNT2 and hCNT3. Apparent affinities (K50), predicted maximum flux values (Vmax) and Hill coefficients (n) were determined from Na+ concentration response curves (0–100 mM NaCl, pH 8.5) in oocytes producing hCNT1, hCNT2 or hCNT3 measured at a 14C-uridine concentration of 20 µM (A–2C). Values were obtained from curve fits to mediated averaged data from 10–12 oocytes, and are presented as means±SE (standard error of the fitted estimate).
Figure 3. Voltage-dependence of hCNT1, hCNT2 and hCNT3 cation activation kinetics. Na+ concentration-response curves (pH 8.5) measured in single representative hCNT1- (A, B), hCNT2- (C, D) and hCNT3- (E, F) producing oocytes at membrane potentials of −30 (A, C, E) and −90 (B, D, F) mV (20 µM uridine). Currents at each Na+ concentration were normalized to the fitted Imax value for that oocyte. Imax values ranged from 45–101 nA. No currents were observed in control water-injected oocytes. Mean Na+-activation data for hCNT1, hCNT2, and hCNT3 is summarized in . The insets are Hill plots of the data.
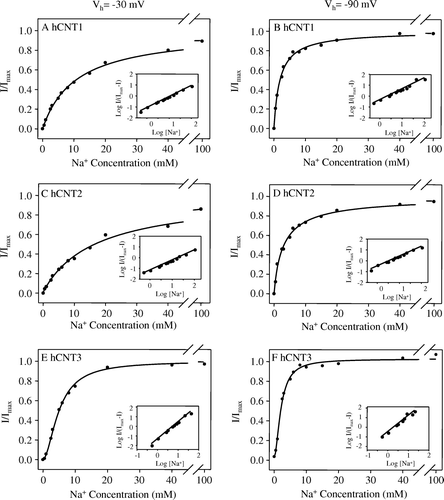
Table II. Voltage-dependence of Na+ activation kinetics of hCNT1, hCNT2 and hCNT3. Apparent affinities (K50) and Hill coefficients (n) for Na+ were determined from Na+ concentration response curves (0–100 mM NaCl, pH 8.5) in oocytes producing hCNT1, hCNT2 or hCNT3 measured at a uridine concentration of 20 µM and membrane potentials of −30 and −90 mV (see for representative hCNT1-, hCNT2-, or hCNT3-producing oocytes). Values were obtained from fits to data from individual oocytes normalized to the fitted Imax value obtained for that cell, and are presented as means±SEM. The numbers in parentheses denote the number of oocytes.
Figure 4. Uridine coupling ratios of hCNT1, hCNT2 and hCNT3. (A) Representative example of the current generated during application of 200 µM 14C-uridine to an hCNT2-producing oocyte in Na+-containing transport medium (100 mM NaCl, pH 8.5) at a membrane potential of −90 mV. Integration of the uridine-evoked current over the uptake period (2 min) yielded the charge moved which was converted to pmol and plotted against radiolabeled uridine uptake (pmol) in the same oocyte. The experiment was repeated in 12 different hCNT2-producing oocytes (C) Corresponding charge-to-14C-uridine uptake ratio plots are also shown for hCNT1 (B, n=11) and hCNT3 (D, n=12) (100 mM NaCl, pH 8.5; Vh= − 90 mV). Linear regression analysis of the data for each plot is indicated by the solid line. The dashed line indicates a theoretical 1:1 charge/flux ratio in (B and C) and a 2:1 charge/flux ratio in (D). Lines were fitted through the origin. Stoichiometries (±SE) obtained from these data are given in .
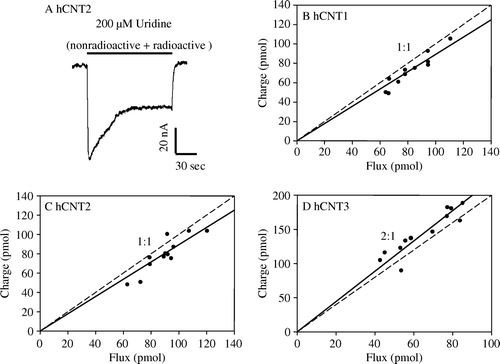
Figure 5. Adenosine coupling ratios of hCNT2 and hCNT3. Charge to 14C-adenosine uptake ratio plots were generated at a membrane potential of −90 mV in Na+-containing transport media (100 mM NaCl, pH 8.5) in hCNT2- (A, n=8) and hCNT3- (B, n=10) producing oocytes. The time of exposure of oocytes to 14C-adenosine (200 µM) was 2 min. Integration of the adenosine-evoked current was used to calculate the net cation influx (charge) and was correlated to the net 14C-adenosine influx (flux). Linear regression analysis of the data for each plot is indicated by the solid line. The dashed line indicates a theoretical 1:1 charge/flux ratio in (A) and a 2:1 charge/flux ratio in (B). Lines were fitted through the origin. Stoichoimetries (±SE) obtained from these data are indicated in .
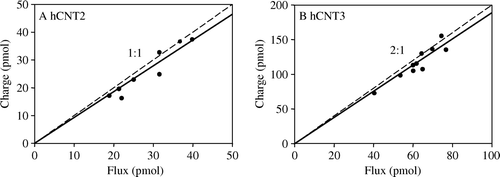
Table III. Stoichiometry of hCNT1, hCNT2 and hCNT3. Charge to 14C-nucleoside ( and ; 100 mM NaCl, pH 8.5) and charge to 22Na+ (data not shown; 1 mM NaCl, pH 8.5) uptake ratio plots were generated at a membrane potential of −90 mV in hCNT1-, hCNT2- and hCNT3-producing oocytes. The numbers in parentheses denote the number of oocytes.
