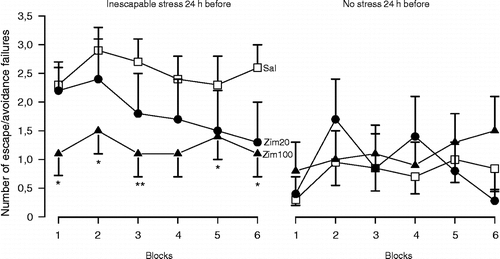
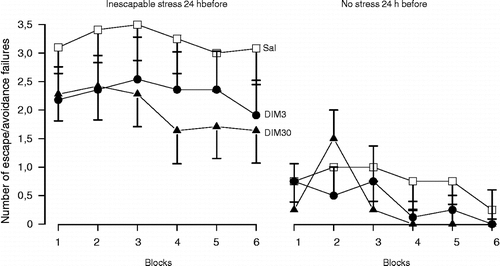
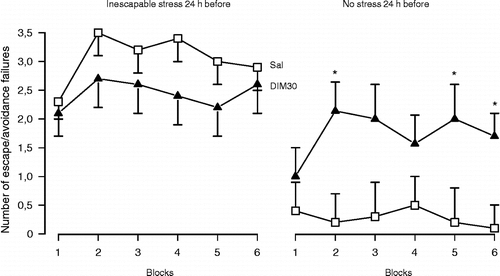
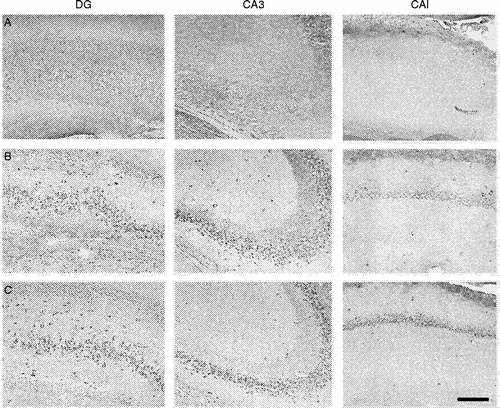
Figures & data
Table I. Gene expression changes in the hippocampal formation induced by stress.
Table II. Gene expression changes in the hippocampal formation induced by antidepressant treatments.