Figures & data
Figure 1 (A) Sleep profile (hypnogram) for a typical night of sleep, showing the nocturnal sequence of electrophysiologically defined sleep stages (REM sleep and Non-REM sleep stages S1–S4). The deepest sleep stages S3 and S4 indicate SWS, characterized by slowly oscillating activity ( < 4 Hz) in the EEG. SWS and REM sleep are not equally distributed across the night. While sleep in the first half of the night (left from vertical line) is dominated by SWS, sleep in the second half of the night (right from vertical line) contains high amounts of REM sleep. W, wake; M, movement activity. (B) Due to circadian and sleep-dependent regulation (see text), cortisol release is strongly suppressed during early sleep, but increases distinctly during late sleep, reaching a maximum in the early morning hours. Accordingly, SWS-dominated early is accompanied by minimal levels of circulating cortisol, while basal cortisol levels are high during REM sleep-dominated late sleep. These changing patterns of electrophysiological and endocrine activity across nocturnal sleep interactively affect memory consolidation.
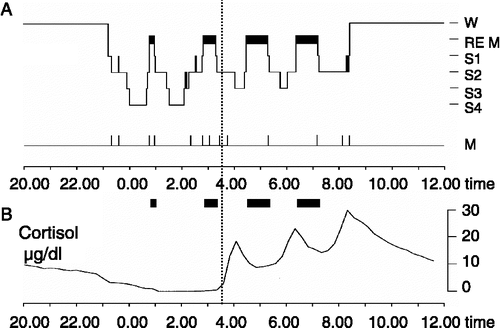
Figure 2 (A) Top: Experimental design for studying the influence of cortisol on memory consolidation during early nocturnal sleep, illustrated by an individual sleep profile. Before sleep, subjects learned memory tasks to a criterion. Lights were turned off at 23.00 h to enable a 3-h period of SWS-rich early sleep. Recall was tested 15–30 min after awakening. Infusion of cortisol (vs. placebo) started at 23.00 h and was discontinued after 2.5 h. Bottom: Mean( ± SEM) plasma cortisol concentrations during the Placebo (dotted line) and Cortisol conditions (solid line). Note that in the cortisol condition plasma concentrations of the hormone were enhanced only during the period of retention sleep, but did not differ from placebo at recall testing. (B) Cortisol infusion during consolidation sleep, compared with placebo, impaired retention of hippocampus-dependent declarative memories for word pairs across sleep (top), but did not affect speed in procedural mirror tracing skills not relying on the hippocampus (bottom). n = 14. *p < 0.05, **p < 0.01. Data from Plihal and Born (Citation1999b).
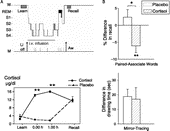
Figure 3 (A) Mean( ± SEM) plasma cortisol concentrations following oral administration of the cortisol synthesis inhibitor metyrapone (3 g, solid line) and placebo (dotted line) before an 8-h period of nocturnal retention sleep (shaded area). Memory tasks (neutral and emotional texts, mirror tracing) were learned before sleep. Retrieval was tested at 11.00 h the next day. (B) Metyrapone impaired retention (percent with reference to performance at learning before sleep) of hippocampus-dependent declarative memories for neutral texts across sleep (left), but augmented amygdala-dependent emotional enhancement in declarative memory, i.e. the superiority of emotional as compared to neutral text recall (middle). Consolidation of procedural memories for mirror tracing was unaffected by the treatment (right). n = 14. *p < 0.05, **p < 0.01, ***p < 0.001. Data from Wagner et al. (Citation2005).
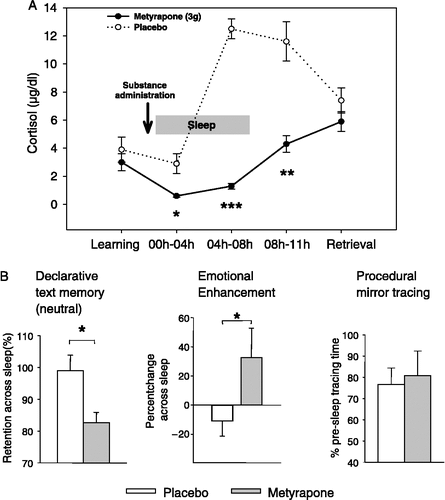
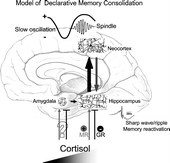
Figure 4 Model of declarative memory consolidation during sleep. During wakefulness information is encoded into neocortical networks and in part in hippocampal networks (gray arrow). During SWS, newly encoded information in the hippocampus is repeatedly reactivated. Reactivations are accompanied by hippocampal sharp wave-ripples. They are driven by slow oscillations which originate in neocortical networks (preferentially in those that were used for encoding at learning) and synchronize hippocampal memory reactivation with the occurrence of spindle activity in thalamo-cortical circuitry. Hippocampal reactivation stimulates a transfer of the newly encoded information back to neocortical networks (black arrow). The hippocampal input arriving in synchrony with spindle input at neocortical circuitry can induce long-term plastic changes selectively at those synapses previously used for encoding, thereby forming a long-term memory of the information in neocortical networks. High concentrations of cortisol inhibit hippocampal memory reactivation and transfer to neocortex via activation of GR. Likewise, memory reactivation and transfer is suppressed in the hippocampus with insufficient occupation of MR. The amygdala enhances hippocampal memory consolidation, depending on the emotional impact of the learning material. As a result of this amygdalar modulation, emotional material is generally better remembered than neutral material. Whether glucocorticoids affect emotional memory formation via an influence on amygdalar function or primarily via hippocampal function is not yet clear.