Figures & data
Figure 1. Stages of Candida albicans biofilm formation and development. Candida albicans biofilm formation is a multifactorial process that consists of four main stages. (A) Initial attachment of planktonic cells: C. albicans yeasts attach to a surface (e.g. epithelia, biomaterials or cellular aggregates) through adhesins such as Als family members. (B) Proliferation and filamentation: yeasts transition to hyphae and this process is regulated by many transcription factors (TFs) including Tec1p and Efg1p. Hyphae express specific adhesins such as Hwp1p and Hyr1p. (C) Biofilm maturation and extracellular matrix formation: the matrix forms around the C. albicans cells, positively regulated by the TF Rlm1p, providing structural support and protection against antifungals and the host immune system. Adhesion is maintained and amino acid metabolism is increased in the biofilm. (D) Biofilm dispersion: yeast cells disperse from the biofilm to colonise other parts of the body. These cells differ from initial planktonic cells as they are more virulent and more likely to form biofilms. Figure created with Adobe Illustrator.
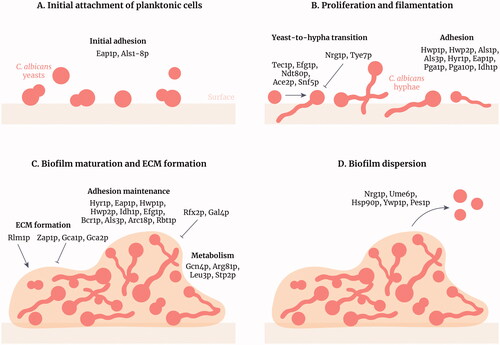
Table 1. Transcription factors that contribute to C. albicans biofilm formation, development, and regulation.
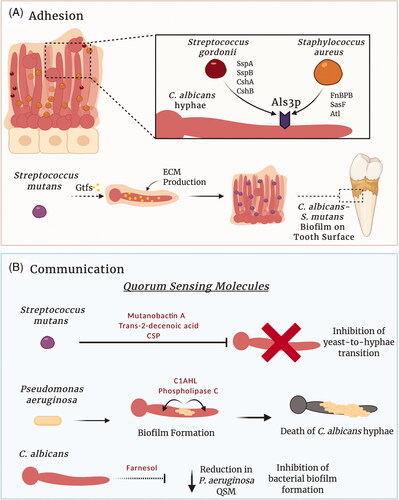
Figure 2. Candida albicans interactions within a multispecies biofilm. Complex physical and chemical interactions govern the development of polymicrobial biofilms. (A) Several factors influence C. albicans–bacterial adhesion. Staphylococcus aureus and Streptococcus gordonii can utilise C. albicans adhesins to directly bind to hyphae. In contrast, glycosyltransferases (Gtfs) secreted by Streptococcus mutans within the oral cavity can bind to C. albicans mannans, increasing the production of glucans and ECM. Consequently, the glucan increases the ability of the bacterium to bind to C. albicans and forms a C. albicans–S. mutans biofilm on the tooth surface (dental plaque). (B) Signalling molecules produced by C. albicans and bacterial species enable inter-kingdom communication within multispecies biofilms. For example, S. mutans and Pseudomonas aeruginosa can secrete quorum sensing molecules that influence the behaviour of C. albicans within the biofilm. Likewise, the C. albicans quorum sensing molecule farnesol, can influence the behaviour of interacting bacteria. Figure created with BioRender.