Figures & data
Figure 1. Characteristic PCNSL imaging pattern on magnetic resonance imaging. A) T1 sequence with gadolinium contrast demonstrates enhancing brain lesion. B) Fluid-attenuated inversion recovery sequence visualizes a mass edema surrounding the mass lesion. C) Diffusion-weighted imaging demonstrates restricted diffusion within the tumor lesions. Abbreviation: PCNSL, primary central nervous system lymphoma.
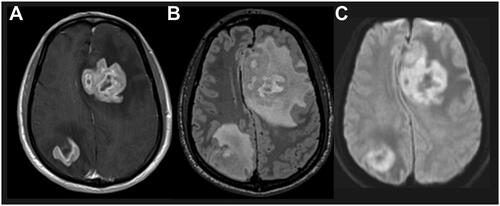
Figure 2. Treatment flow for patients with newly diagnosed PCNSL. A. Frontline therapy for PCNSL for fit patients includes HD-MTX regimens as induction therapy, with ASCT consolidation therapy. Fitness is determined by a combination of age, comorbidities, organ function, frailty, risk of having neurotoxic effects from treatment, performance status, and patient priorities. For patients who are not deemed fit for this therapy, alternative induction and consolidation strategies include non-myeloablative therapies, WBRT, or ifosamide- or cytarabine-containing regimens. B. For patients with R/R PCNSL, treatment options are guided by the previous therapy received and the length of time to progression from prior therapy. aWBRT should be used with caution due to neurotoxicity concerns, especially in elderly patients or in combination with chemotherapy. bHD-MTX regimens typically include maximum tolerated dose with an alkylating agent with or without rituximab and with or without high-dose cytarabine. cFor patients for whom consolidation therapy with ASCT is not recommended, potential consolidation strategies include nonmyeloablative high-dose chemotherapy alone or with etoposide, or maintenance therapy with temozolomide, rituximab, or MTX. dSalvage therapy may include retreatment with HD-MTX with or without rituximab or ibrutinib; ibrutinib, temozolomide, rituximab with or without temozolomide; lenalidomide with or without rituximab; high-dose cytarabine; high-dose ifosfamide; or pemetrexed; patients with R/R PCNSL are recommended to be enrolled into clinical trials if eligible. Adapted from information in [Citation4, Citation7, Citation13, Citation14]. Abbreviations: ASCT, autologous stem cell therapy; BSC, best supportive care; CR, complete response; CT, chemotherapy; HD, high-dose; HD-MTX, high-dose methotrexate; mo, months; PCNSL, primary central nervous system lymphoma; PR, partial response; R/R, relapsed/refractory; RT, radiotherapy; SD, stable disease; TTP, time to progression; WBRT, whole-brain radiotherapy.
![Figure 2. Treatment flow for patients with newly diagnosed PCNSL. A. Frontline therapy for PCNSL for fit patients includes HD-MTX regimens as induction therapy, with ASCT consolidation therapy. Fitness is determined by a combination of age, comorbidities, organ function, frailty, risk of having neurotoxic effects from treatment, performance status, and patient priorities. For patients who are not deemed fit for this therapy, alternative induction and consolidation strategies include non-myeloablative therapies, WBRT, or ifosamide- or cytarabine-containing regimens. B. For patients with R/R PCNSL, treatment options are guided by the previous therapy received and the length of time to progression from prior therapy. aWBRT should be used with caution due to neurotoxicity concerns, especially in elderly patients or in combination with chemotherapy. bHD-MTX regimens typically include maximum tolerated dose with an alkylating agent with or without rituximab and with or without high-dose cytarabine. cFor patients for whom consolidation therapy with ASCT is not recommended, potential consolidation strategies include nonmyeloablative high-dose chemotherapy alone or with etoposide, or maintenance therapy with temozolomide, rituximab, or MTX. dSalvage therapy may include retreatment with HD-MTX with or without rituximab or ibrutinib; ibrutinib, temozolomide, rituximab with or without temozolomide; lenalidomide with or without rituximab; high-dose cytarabine; high-dose ifosfamide; or pemetrexed; patients with R/R PCNSL are recommended to be enrolled into clinical trials if eligible. Adapted from information in [Citation4, Citation7, Citation13, Citation14]. Abbreviations: ASCT, autologous stem cell therapy; BSC, best supportive care; CR, complete response; CT, chemotherapy; HD, high-dose; HD-MTX, high-dose methotrexate; mo, months; PCNSL, primary central nervous system lymphoma; PR, partial response; R/R, relapsed/refractory; RT, radiotherapy; SD, stable disease; TTP, time to progression; WBRT, whole-brain radiotherapy.](/cms/asset/6306ae4c-9178-4431-9779-963c5387a15c/ilal_a_2342560_f0002_c.jpg)
Table 1. Selected agents in recent trials for PCNSL by target or MOA.
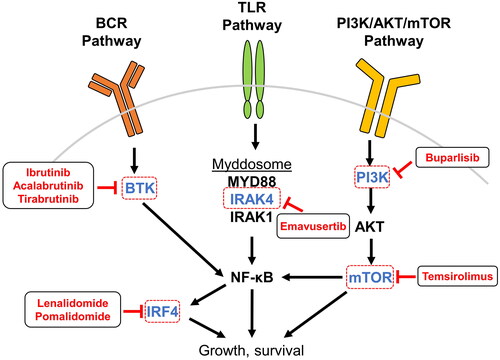
Figure 3. Inhibition of PCNSL-associated signaling pathways. PCNSL is frequently driven by overactivation of pathways leading to NF-κB, and many therapeutic agents in use and development for patients with PCNSL target components upstream and downstream of NF-κB. BCR signaling to NF-κB is transduced by BTK and is targeted by the small-molecule inhibitors ibrutinib, acalabrutinib, and tirabrutinib. TLR activation by ligand binding leads to assembly and activation of the myddosome protein complex that incorporates IRAK4, IRAK1 phosphorylation, and downstream activation of NF-κB; the small molecule emavusertib inhibits IRAK4. Within the PI3K pathway, mTOR is inhibited by temsirolimus and PI3K by the pan-PI3K-inhibitor buparlisib. NF-κB itself regulates the expression of IRF4, which is a target of the IMiDs lenalidomide and pomalidomide. Abbreviations: AKT, protein kinase B; BCR, B-cell receptor; BTK, Bruton’s tyrosine kinase; IMiD, immunomodulatory imide drugs; IRAK, interleukin-1 receptor-associated kinase; IRF4, interferon regulatory factor 4; mTOR, mammalian target of rapamycin; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; PI3K, phosphatidylinositol-3-kinase; TLR, toll-like receptor.