Figures & data
Figure 1. GLX7013159 treatment decreases blood glucose levels and water consumption in athymic diabetic mice with human islet transplants.
(A) Average blood glucose values for GLX7013159-treated and control mice, following alloxan injection on day 0, and human islet transplantation on days 2 and 3. Values of HI (>27.8 mmol/L) were registered as 27.8 mmol/L. Mean AUC (post-transplantation) for control and GLX7013159-treated mice (GLX mice) were 752 ± 8 and 684 ± 21 mmol/L × 30 days, respectively (p = 0.01, n = 7–8 animals in each group). All values are given as means ± SEM. (B) Water consumption was lower throughout the treatment period for GLX7013159-treated mice, with AUC 1113 ± 83 mL for control and 888 ± 51 mL for the treatment group (p < 0.05). All values are given as means ± SEM.
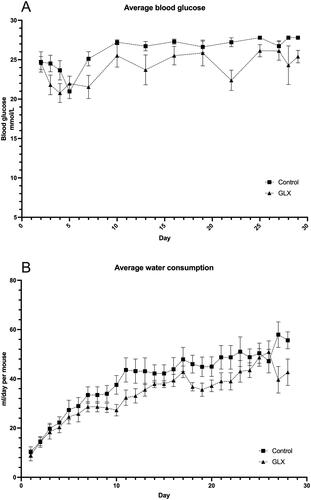
Figure 2. GLX7013159-treated mice display higher insulin levels 10 min post IVGTT and higher human C-peptide.
(A) An intravenous glucose tolerance test (2g glucose/kg BW) was conducted 1-2 days prior to end of the experiment. Blood samples were withdrawn 10 minutes after glucose injection. Mice receiving GLX7013159 (GLX) had higher serum insulin levels compared to control mice (p < 0.05 using Student’s unpaired t-test; n = 7–8 animals in each group). All values are given as means ± SEM. (B) Plasma collected at the termination of the experiment was analyzed using an ultrasensitive human C-peptide ELISA (Mercodia), GLX7013159-treated mice (GLX) had higher levels of human C-peptide compared to controls (p < 0.05 using Student’s unpaired t-test; n = 7–8 animals in each group). All values are given as means ± SEM.
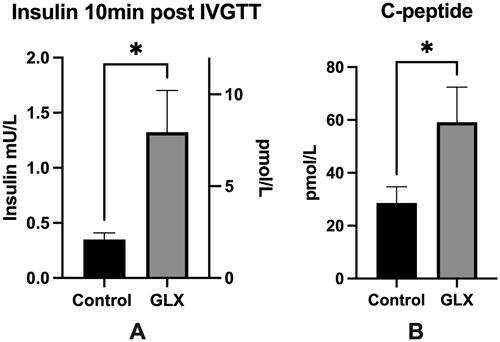
Figure 3. GLX70131159-treated mice grafts are more responsive to glucose during perifusion. Directly after collection, the transplanted islet grafts were subjected to a glucose perifusion analysis using KRBH containing 2 mmol/L and 20 mmol/L glucose. Buffer samples were collected at multiple time points and analyzed for human insulin using an ultrasensitive insulin ELISA (Mercodia). Stimulation indexes were calculated by dividing the AUCs of high glucose perifusion with low glucose perifusion for each islet graft. Grafts from GLX7013159 treated mice (GLX) displayed a higher stimulation index compared to control grafts (p < 0.05 using Student’s unpaired t-test; n = 7–8 grafts in each group). All values are given as means ± SEM.
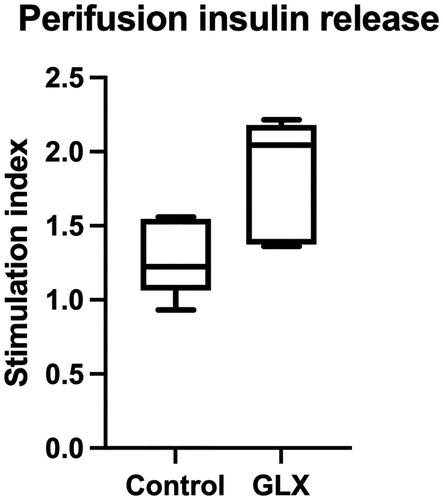
Table 1. Characteristics of the novel NOX inhibitor GLX7013159 compared to the previously described highly selective NOX4 inhibitor GLX7013114. NA: not active.
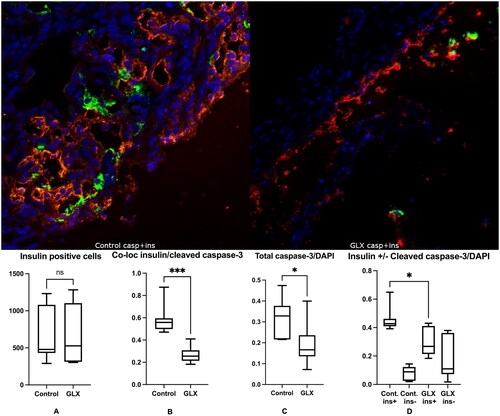
Figure 4. GLX7013159 treatment decreased cleaved caspase-3 (green) expression in insulin-positive cells (red). Top: The retrieved islet grafts were sectioned and stained for Insulin (RED), Cleaved caspase-3 (GREEN) and DAPI (BLUE). Top left shows a section from a control mouse, whereas top right shows a section from a GLX7013159-treated mouse. Bottom: Immunohistochemistry was assessed both by manually counting, by an investigator not aware of the identity of the sections, of the number of cells positive for insulin and/or cleaved caspase 3, and by measuring signal intensity using the ImageJ software. GLX7013159 treatment did not affect the total amount of insulin-positive cells (A, y-axis = number of cells per graft), but resulted in a marked decrease of cells co-expressing cleaved caspase 3 and insulin when manually counted (B, p < 0.001). The total cleaved caspase-3/DAPI signal intensity was lower in GLX7013159 treated mice (C, p < 0.05), GLX7013159 treated mice expressed lower cleaved caspase-3 signaling in insulin-positive areas (D, p = 0.01), no difference was observed in insulin negative areas (D). All images were analyzed blindly. All comparisons were performed by Student’s unpaired t test. Values are given as means ± SEM for 7–8 grafts in each group.