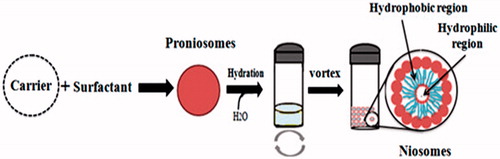
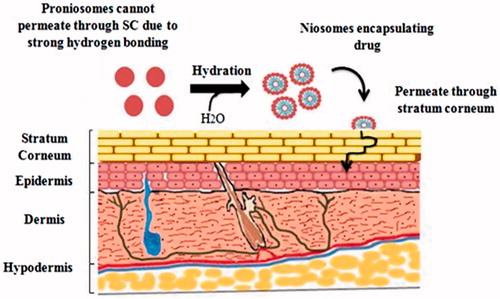
Figures & data
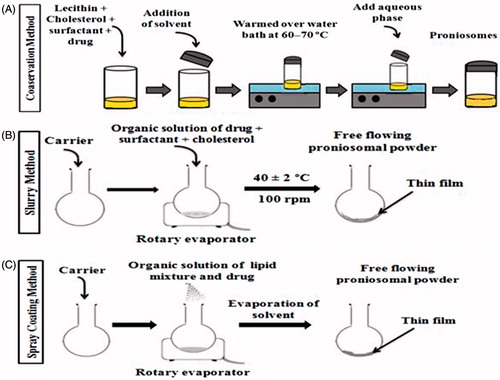
Table 1. Description of preparation methods, their principle and type of formulation formed.
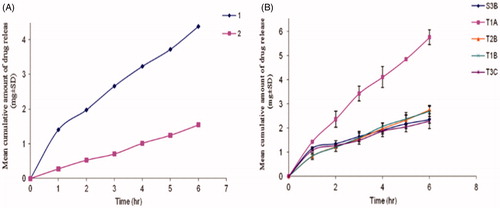
Table 2. Outline of drug delivery applications of proniosomes through different routes, composition and their in vitro/in vivo effects.