Figures & data
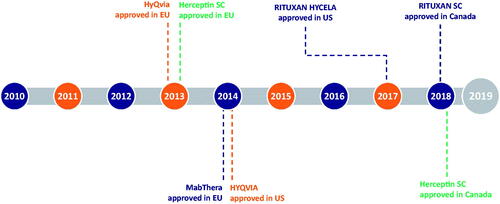
Figure 1. rHuPH20 mechanism of action. (A) Hyaluronan creates a resistance to bulk fluid flow and limits large volume SC drug delivery, dispersion, and absorption. (B) rHuPH20 depolymerizes hyaluronan, (C) facilitating SC bulk fluid flow and increasing the dispersion and absorption of co-administered therapeutics.
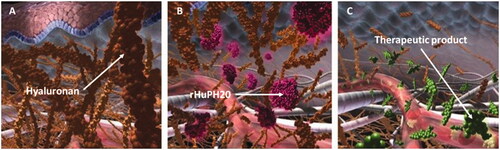
Figure 2. Timeline of rHuPH20 partner product approvals. HyQVIA/HYQVIA, human immunoglobulin infusion (Immune Globulin 10%) and rHuPH20; Herceptin SC, trastuzumab and rHuPH20; MabThera SC/RITUXAN HYCELA/RITUXAN SC, rituximab and rHuPH20. Rituximab and trastuzumab were the first and second monoclonal antibodies approved for the treatment of cancer, respectively (Dillman,Citation1999; Pierpont et al.,Citation2018), and the first to undergo IV to SC conversion.