Figures & data
Table 1. Full factorial design (21, 51) used for optimization formulations showing independent, dependent variables (responses) and desired outcomes.
Table 2. Composition of DOM-loaded ethosomal suspensions.
Table 3. Composition of prepared gels loaded DOM-ethosomal suspension.
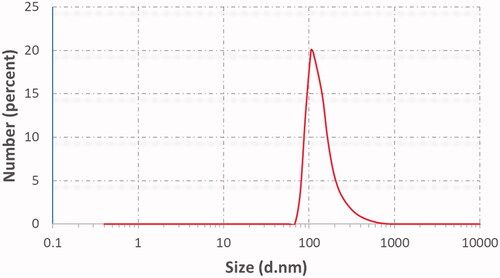
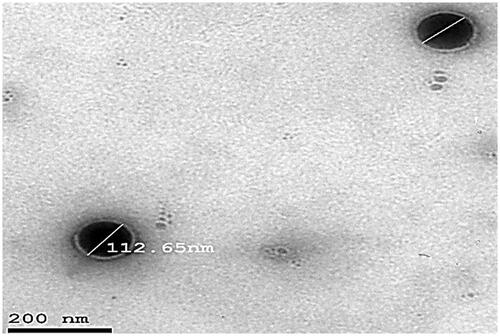
Table 4. Vesicular size, PDI, zeta potential (ZP), EE%, and % drug released after 6 h (Q6h) of DOM-loaded ethosomal suspension (mean ± SD, n = 3).
Figure 1. In vitro release profiles of DOM solution and different DOM-loaded ethosomes formulations in phosphate buffer saline (pH 6.8) at 37 °C ± 0.5 °C.
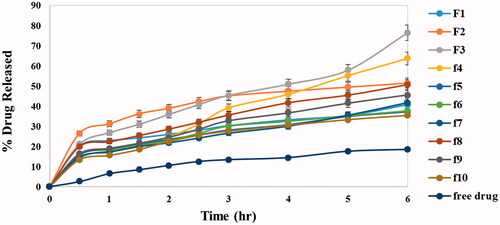
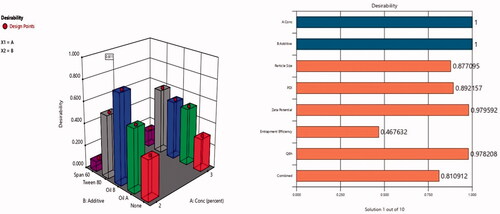
Figure 2. Response bar plots for the effect of lecithin concentration (%) (X1) and additives (X2) on (A) PS, (B) ZP, (C) %EE, and (D) Q6h on DOM-loaded ethosomes.
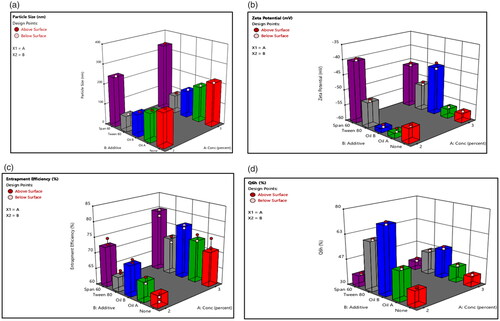
Table 5. Output data of the (21, 51) full factorial analysis of the formulas, predicted and actual values for the optimum formulation (F3).
Table 6. The actual results of optimum formulation against the suggested one.
Table 7. Effect of storage on properties of selected formulation (F3).
Figure 8. Ex vivo permeation profiles of DOM from prepared ethosomal suspension (F3) compared to pure drug.
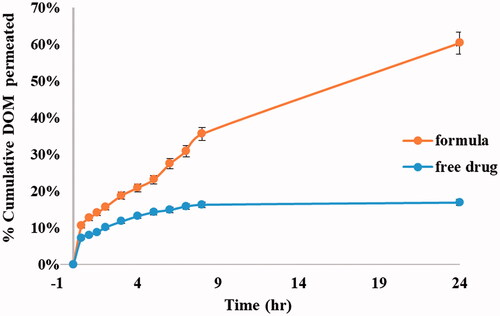
Figure 9. Rheological behavior of (a) (F11) ethosomal rectal gel (0.5% Carbopol), (b) (F12) ethosomal rectal gel (1% Carbopol), (c) (F13) free drug rectal gel 0.5% Carbopol, and (d) (F14) free drug rectal gel (1% Carbopol).
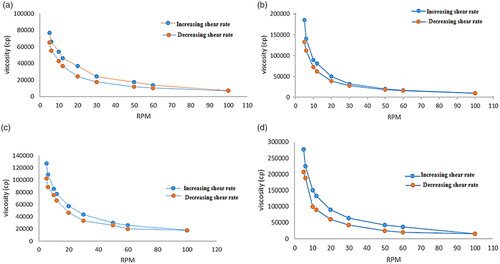
Figure 10. Mean DOM plasma concentration-time curves in rats after administration of a single dose (3.1 mg/kg) of DOM-loaded ethosomal gel (F11) and pure DOM gel (F13).
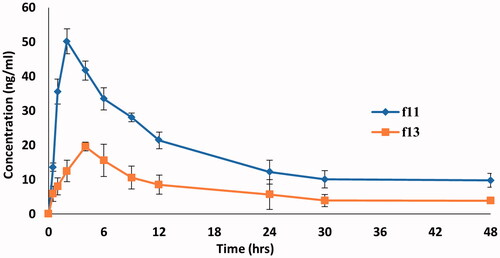
Table 8. Mean pharmacokinetics parameters of DOM following single rectal administration for DOM-loaded ethosomal gel (F11) and pure DOM gel (F13).
Data availability statement
All data and materials are available.