Figures & data
Table 1. Draper-Lin small composite design (DLD) independent and dependent variables with the chosen responses and assigned goals of MTC–loaded lipidic nano-vesicular hybrids.
Table 2. Experimental runs and observed values of responses for DLD MTC–loaded lipidic nano-vesicular hybrids, along with the two optimized formulations (Opti-Max and Opti-Min MTC–loaded PEG-T-Chito-Lip nano-vesicular formulations).Table Footnotea
Figure 1. Schematic illustration of the performed valuation of nose-to-brain delivery and in-vivo pharmacokinetics of the intranasal dual-optimized MTC–loaded PEG-T-Chito-Lip nano-vesicular ISG. Abbreviations: MTC, metoclopramide hydrochloride; PEG-T-Chito-Lip nano-vesicular hybrid, PEGylated Tween 80–functionalized chitosan–lipidic nano-vesicular hybrid; ISG, in-situ gel; I.V., intravenous.
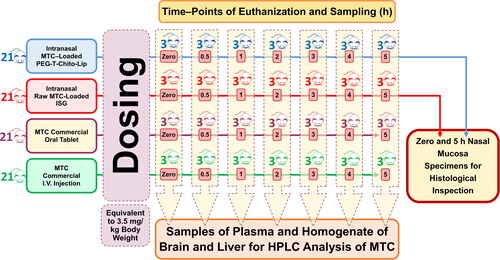
Figure 2. In-vitro release profile of MTC from different DLD formulations, Opti-Max and Opti-Min MTC–loaded PEG-T-Chito-Lip nano-vesicular formulations. Abbreviations: MTC, metoclopramide hydrochloride; PEG-T-Chito-Lip nano-vesicular hybrid, PEGylated Tween 80–functionalized chitosan–lipidic nano-vesicular hybrid.
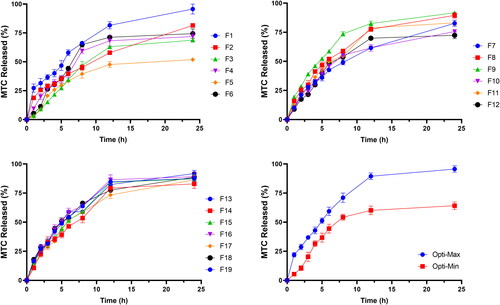
Table 3. Kinetics studies of the release profiles of MTC from different DLD formulations, as well as the two optimized formulations (Opti-Max and Opti-Min MTC–loaded PEG-T-Chito-Lip nano-vesicular formulations).
Table 4. Estimated effects of factors and associated p-values for responses.
Figure 3. Pareto chart showing the standardized effects of factors on the observed responses (Y1–Y5).
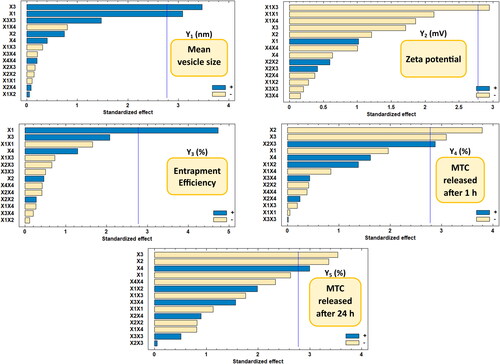
Figure 4. Contour-response surface plot showing the effects of factors on the observed responses (Y1–Y5).
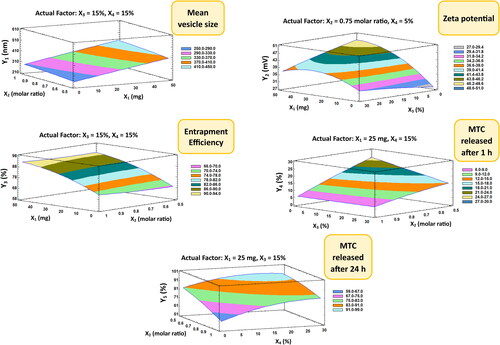
Figure 5. Light (left figures) and TEM (right figures) micrographs of the Opti-Max (A and B) and Opti-Min (C and D) MTC–loaded PEG-T-Chito-Lip nano-vesicular formulations. Abbreviations: MTC, metoclopramide hydrochloride; PEG-T-Chito-Lip nano-vesicular hybrid, PEGylated Tween 80–functionalized chitosan–lipidic nano-vesicular hybrid.
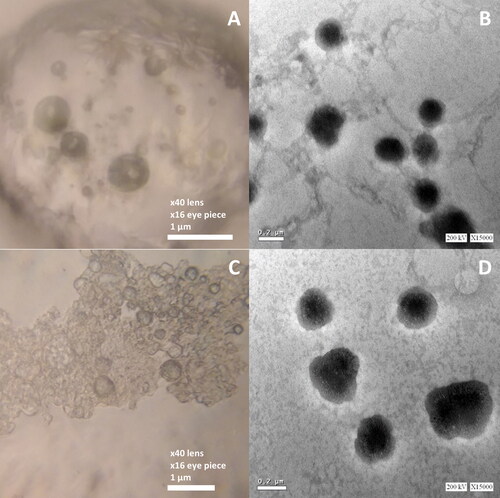
Figure 6. DSC/TGA thermograms of the pure MTC, Opti-Min and Opti-Max MTC–loaded PEG-T-Chito-Lip nano-vesicular formulations. Abbreviations: MTC, metoclopramide hydrochloride; PEG-T-Chito-Lip nano-vesicular hybrid, PEGylated Tween 80–functionalized chitosan–lipidic nano-vesicular hybrid.
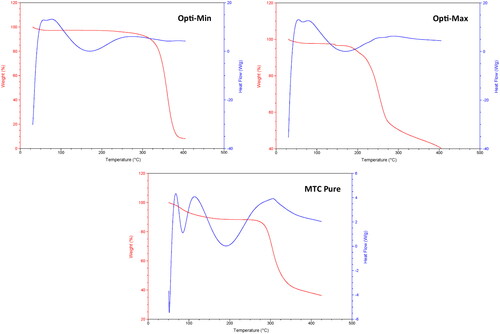
Figure 7. Plasma, brain and liver MTC concentration vs time profiles after administration of the intranasal dual-optimized MTC–loaded PEG-T-Chito-Lip nano-vesicular ISG, intranasal raw MTC-loaded ISG, MTC commercial oral tablet, and MTC commercial I.V. injection, in Sprague Dawley rats (n = 3). The dose of MTC in all formulations was equivalent to 3.5 mg/kg body weight. *, #, and $indicate P < 0.05 versus intranasal raw MTC-loaded ISG, MTC commercial oral tablet, and MTC commercial I.V. injection, respectively. Abbreviations: MTC, metoclopramide hydrochloride; PEG-T-Chito-Lip nano-vesicular hybrid, PEGylated Tween 80–functionalized chitosan–lipidic nano-vesicular hybrid; ISG, in-situ gel; I.V., intravenous.
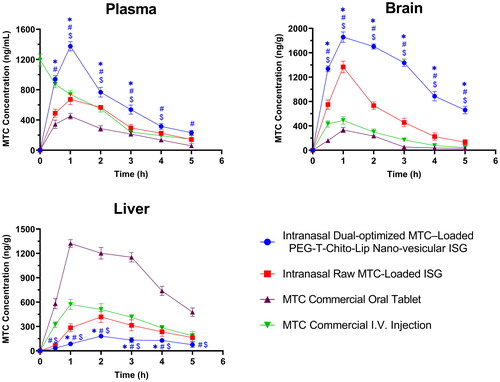
Table 5. The PK parameters of MTC in the plasma, brain and liver after administration of the intranasal dual-optimized MTC–loaded PEG-T-Chito-Lip nano-vesicular ISG, intranasal raw MTC-loaded ISG, MTC commercial oral tablet, and MTC commercial I.V. injection, in Sprague Dawley rats (n = 3).
Table 6. The absolute and relative bioavailability parameters of MTC in the plasma after administration of the intranasal dual-optimized MTC–loaded PEG-T-Chito-Lip nano-vesicular ISG and intranasal raw MTC-loaded ISG in Sprague Dawley rats (n = 3).
Figure 8. Evaluation of the nose-to-brain delivery of the dual-optimized MTC–loaded PEG-T-Chito-Lip nano-vesicular ISG formulation. a; MTC Brain/Blood ratio vs time of the intranasal dual-optimized MTC–loaded PEG-T-Chito-Lip nano-vesicular ISG, intranasal raw MTC-loaded ISG, MTC commercial oral tablet, and MTC commercial I.V. injection, in Sprague Dawley rats (n = 3). b; DTE%, DTP%, AUCbf and DTI of the intranasal dual-optimized MTC–loaded PEG-T-Chito-Lip nano-vesicular ISG and intranasal raw MTC-loaded ISG relative to the MTC commercial I.V. injection, in Sprague Dawley rats (n = 3). The dose of MTC in all formulations was equivalent to 3.5 mg/kg body weight. *, #, and $indicate p < 0.05 versus intranasal raw MTC-loaded ISG, MTC commercial oral tablet, and MTC commercial I.V. injection, respectively. Abbreviations: MTC, metoclopramide hydrochloride; PEG-T-Chito-Lip nano-vesicular hybrid, PEGylated Tween 80–functionalized chitosan–lipidic nano-vesicular hybrid; ISG, in-situ gel; I.V., intravenous; DTE: drug targeting efficiency; AUCbf: the AUC of the brain fraction; DTP: nose-to-brain direct transport percentage; DTI: drug targeting index.
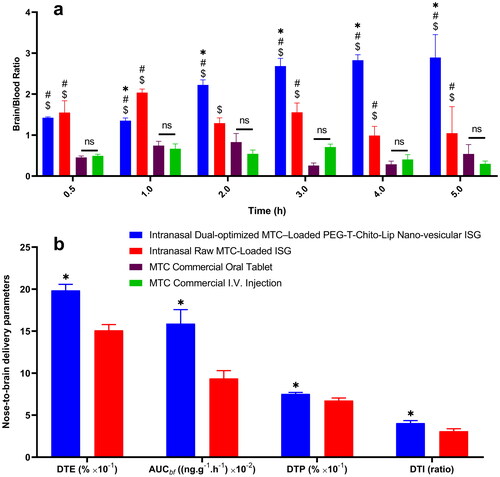
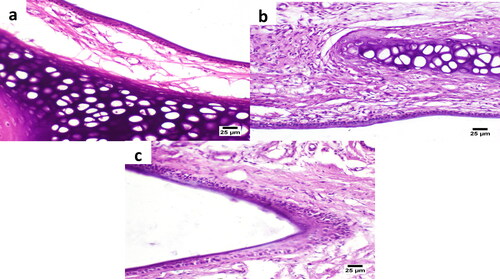
Figure 9. Histological images of the rat nasal mucosa after 5 h treatment with the intranasal dual-optimized MTC–loaded PEG-T-Chito-Lip nano-vesicular ISG (a) and intranasal raw MTC-loaded ISG (b), along with the normal untreated rat nasal mucosa (c) at time zero. The nasal cavity in all images showed apparently normal nasal mucosa (H&E).