Figures & data
Figure 1 Mean concentrations of total radioactivity in plasma and whole blood and of TCA-precipitable radioactivity in plasma following intravenous administration of 125I-PEG-hemoglobin SBl (nominal 5 ml/kg/10 minute infusion rate) to male rats.
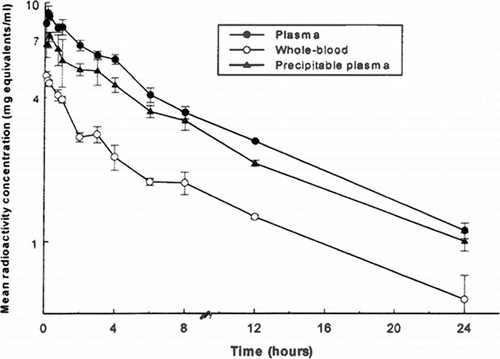
Table 1. Total radioactivity pharmacokinetic data following intravenous administration of 125I-PEG-hemoglobin SBl (nominal 5 ml/kg/10 minute and 12.5ml/kg/25 minute infusion rates) to male rats
Table 2. Whole blood: plasma radioactivity concentration ratios, calculated concentrations of radioactivity in blood cells and distribution of systemic radioactivity into blood cells at the time of sacrifice following intravenous administration of 125I-PEG-hemoglobin SB1 (nominal 5 ml/kg/10 minute infusion rate) to male rats
Table 3. Proportions of the plasma radioactivity precipitated by 15% trichloroacetic acid solution and concentrations of radioactivity in the precipitate following intravenous administration of 125I-PEG-hemoglobin SB1 (nominal 5 ml/kg/10 minute infusion rate) to male rats
Figure 2 Mean concentrations of total radioactivity in plasma and whole blood and of TCA-precipitable radioactivity in plasma following intravenous administration of I25I-PEG-hemoglobin SBl (nominal 12.5 ml/kg/10 minute infusion rate) to male rats.
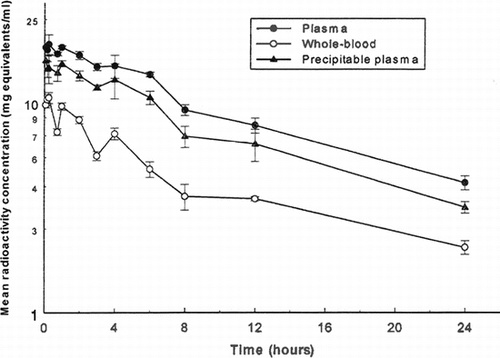
Table 4. Whole blood: plasma radioactivity concentration ratios, calculated concentrations of radioactivity in blood cells and distribution of systemic radioactivity into blood cells at the time of sacrifice following intravenous administration of I25I-PEG-hemoglobin SB1 (nominal 12.5 ml/kg/25 minute infusion rate) to male rats
Table 5. Proportions of the plasma radioactivity precipitated by 15% trichloroacetic acid solution and concentrations of radioactivity in the precipitate following intravenous administration of I25I-PEG-hemoglobin SB1 (nominal 12.5 ml/kg/25 minute infusion rate) to male rats
Figure 3 Mean concentrations of radioactivity in plasma and whole blood following a single intravenous administration of l25I-PEG-hemoglobin SBl (nominal 10 ml/kg infused over 50 minutes) to male dogs.
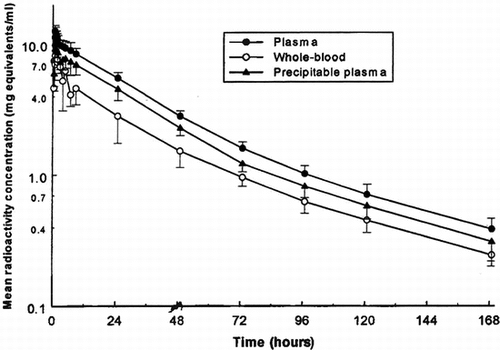
Table 6. Total radioactivity pharmacokinetic data following intravenous administration of 125I-PEG-hemoglobin SB1 (nominal 10 ml/kg infused over 50 minutes) to male dogs
Table 7. Concentrations of radioactivity in plasma precipitated by 15% trichloroacetic acid solution during and after intravenous administration of 125I-PEG-hemoglobin SB1 (nominal 10 ml/kg infused over 50 minutes) to male dogs
