Figures & data
Table 1. Immobilization of trypsin to thermosensitive copolymerFootnote‡
Table 2. Kinetic parameters of free trypsin and conjugates
Figure 1 Effect of pH on enzyme activity. The enzyme assay for free (▴) and immobilized enzyme (conjugate A, •; conjugate B, ○) with matched enzyme activities was carried out at pH values ranging from pH 5.5–10.0 at 25°C with BAPNA as the substrate as given in materials and methods. The relative activity (%) was calculated by taking the maximum activity at optimum pH to be 100% and calculating activities at other pH values relative to it. The experiment was carried out in duplicate and the error bars represent the variation in the readings. The observed standard deviation in each set of readings was less than 0.1%.
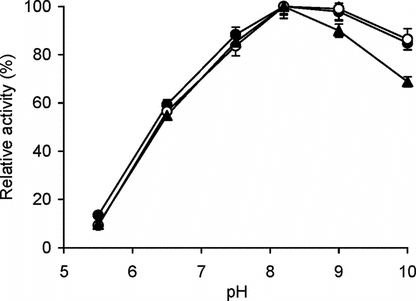
Figure 2 Effect of temperature on enzyme activity. The enzyme assay for free (▴) and immobilized enzyme (conjugate A, •; conjugate B, ○) with matched enzyme activities was carried out at temperatures ranging from 20–70°C with BAPNA as the substrate. The relative activity (%) was calculated by taking the maximum activity at optimum temperature to be 100% and calculating activities at other temperatures relative to it. The experiment was carried out in duplicate and the error bars represent the variation in the readings.
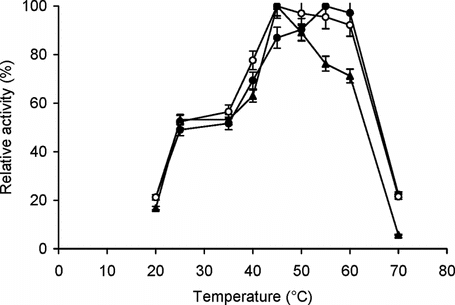
Figure 3 Autolysis of free and immobilized trypsin. Free (▴) and immobilized trypsin (conjugate A, •; conjugate B, ○) [in 0.05 M Tris-HCl, pH 8.0] with matched enzyme activities were incubated at 50°C. Aliquots were taken out at various intervals and assay was carried out with BAPNA (made in 0.05 M Tris-HCl, pH 8.0) after cooling to 25°C. The relative activity (%) was calculated by taking the activity at 0 min to be 100% and calculating activities at other intervals relative to it. The experiment was carried out in duplicate and the error bars represent the variation in the readings. The observed standard deviation in each set of readings was less than 0.1%.
![Figure 3 Autolysis of free and immobilized trypsin. Free (▴) and immobilized trypsin (conjugate A, •; conjugate B, ○) [in 0.05 M Tris-HCl, pH 8.0] with matched enzyme activities were incubated at 50°C. Aliquots were taken out at various intervals and assay was carried out with BAPNA (made in 0.05 M Tris-HCl, pH 8.0) after cooling to 25°C. The relative activity (%) was calculated by taking the activity at 0 min to be 100% and calculating activities at other intervals relative to it. The experiment was carried out in duplicate and the error bars represent the variation in the readings. The observed standard deviation in each set of readings was less than 0.1%.](/cms/asset/f3d56706-4b26-4bda-84c3-d934105381c6/ianb19_a_168360_f0003_b.gif)
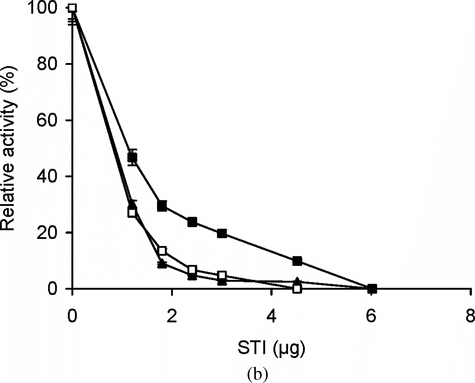
Figure 4 (a) Effect of different concentrations of STI on the activity of free and immobilized trypsin with BAPNA. Free (▴) and immobilized enzyme (conjugate A, •; conjugate B, ○) with matched enzyme activities were incubated with different concentrations of STI for 15 min at 25°C, then trypsin activity was assayed with BAPNA under standard assay conditions. The relative activity was defined as the ratio of activities after incubating with and without STI. The experiment was carried out in duplicate and the error bars represent the variation in the readings. The observed standard deviation in each set of readings was less than 0.1%; (b) Effect of different concentrations of STI on the activity of free and immobilized trypsin with casein. Free (▴) and immobilized enzyme (conjugate A, ▪; conjugate B, □) with matched enzyme activities were incubated with different concentrations of STI for 15 min at 25°C, then trypsin activity was assayed with casein under standard assay conditions at 37°C. The relative activity was defined as the ratio of activities after incubating with and without STI. The experiment was carried out in duplicate and the error bars represent the variation in the readings. The observed standard deviation in each set of readings was less than 0.1%.
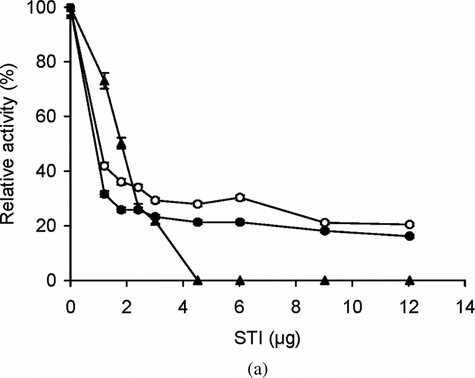
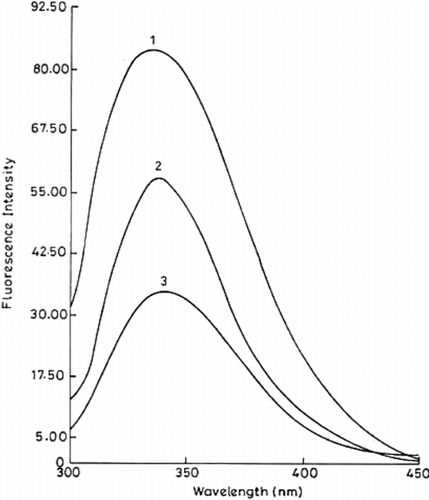
Figure 5 Fluorescence spectra of free and immobilized trypsin. The fluorescence emission spectra of immobilized (conjugate A, curve 1; conjugate B, curve 2) and free trypsin (curve 3) were recorded on a Shimadzu RF-5000 spectrofluorophotometer with a band width of 5 nm and 1 cm path length (at an excitation wavelength of 284 nm). The amount of protein was 12.6 µg in all the cases.