Figures & data
Figure 1 Deployed AneuRx stent-graft manufactured by Medtronic-AVE. The device is made by assembling a bifurcation with asymmetrical limbs with an ipsilateral limb. The thin wall of the woven polyester tubes is externally supported by self–expanding stents made of Nitinol, a shape memory alloy, and sewn by means of a polyester suture. The cross-section investigated is given by the transversal double-headed arrow.
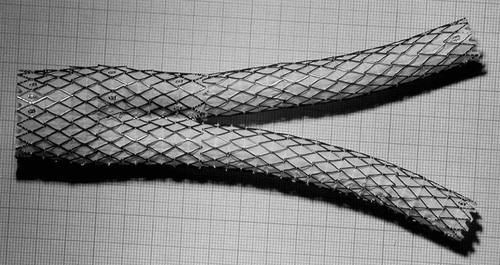
Figure 2 CT Scan of the aneurysm of the abdominal aorta fitted with the AneuRx endovascular prosthesis. The limbs of the device within the aneurysmal sac are well delineated and the content of the sac appears solidified.
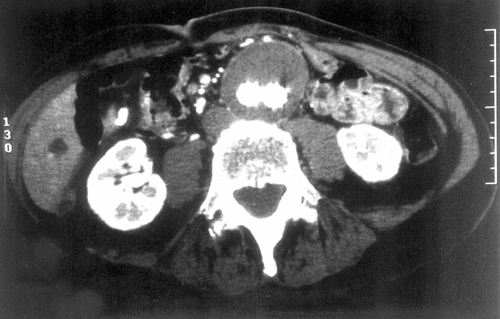
Figure 3 Macroscopic cross-section of the specimen harvested at autopsy. The aortic wall is well delineated but fragmented in several locations. The aneurysmal sac is filled with a solidified substance with a void more or less filled with a gelatinous content below the two limbs of the graft that are well encapsulated.
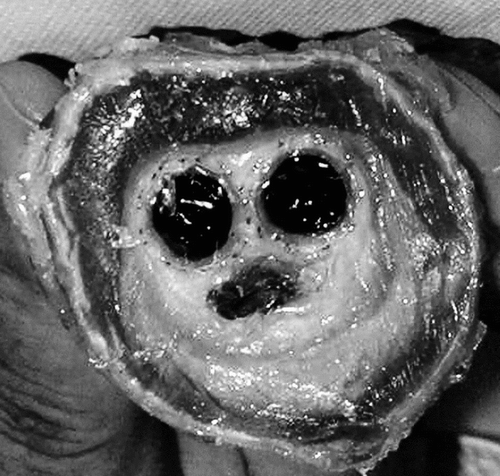
Figure 4 Cross-section of the specimen investigated at low magnification; the aortic wall is poorly defined and presents an irregular and incomplete structure. The cross–sections of the limbs are well identified. The aneurysmal sac is filled with fibrinous deposits almost acellular, which shrank during the fixation process. The void at the bottom is an artifact resulting from shrinkage of the solid filling of the sac where water content was very high (A). Both limbs were surrounded with organized fibrin and collagen deposits (B). The foreign material was well encapsulated, both outside and inside. The external encapsulation was very adhesive to the Nitinol stents and the polyester wall, whereas this internal capsule, as the result of the hyaline content, also shrank.
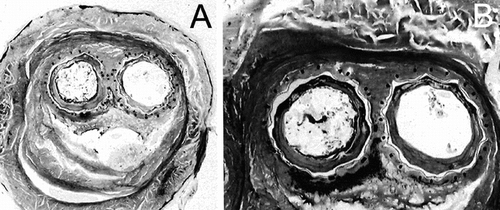
Figure 5 Illustration of the imperviousness of the polyester wall of the prostheses. The construction is very regular and the compaction of the yarns was very well preserved during the implantation. Such a structure does not permit blood percolating through the wall after a thin layer of fibrin is deposited all over the luminal surface.
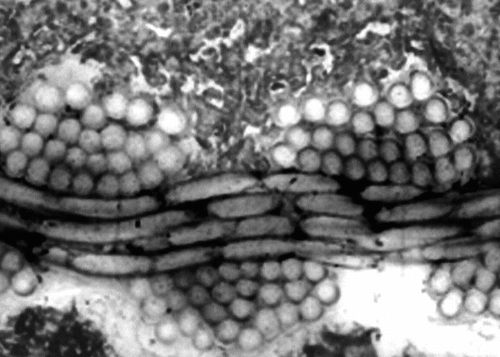
Figure 6 Illustration of the regularity of the polyester wall. Such a structure is very compact and unlikely to be penetrated by cells and fibrin. The weakness is the lack of capacity to securely anchor any encapsulation of fibrous tissues.
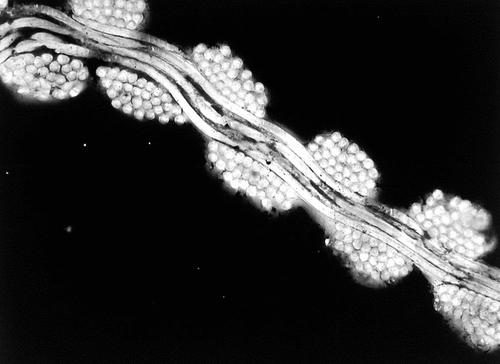
Figure 7 Cross-section at the contact between the ipsilateral limb and the bifurcation sutures are well identified. However, the surfaces of the Nitinol stents present some irregularities.

Figure 8 Absence of exacerbated inflammatory reaction caused by the Nitinol wire. Note that the shape of the wire is ill-defined: it is probably related to an early stage of corrosion.
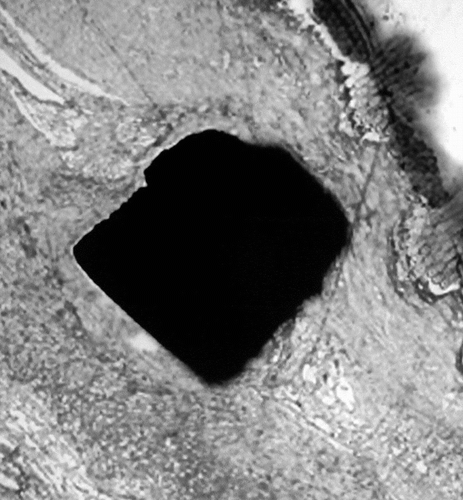
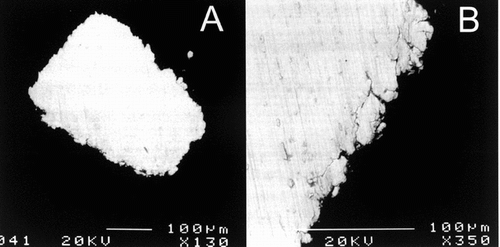
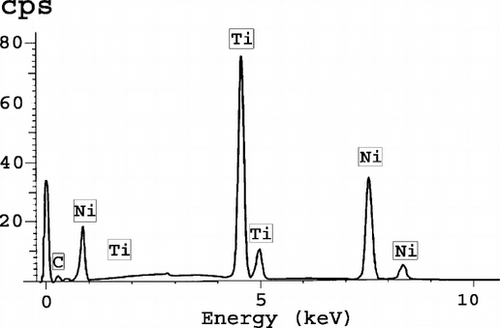
Figure 9 Backscattered observation of the Nitinol wires demonstrating an early stage of corrosion with fragmentation and particle dispersion. The surface of the Nitinol stents is eroded, the material in contact with the tissues appears fragile and some fragments can be dispersed in the surrounding tissues.