Figures & data
Figure 1 Graphical illustration of the components contributing to blood sparing after treatment with HBOC solution. Parameters are plotted for an HBOC dose of two blood equivalent units, an HBOC plasma half-life of one day, and an excess erythropoesis rate of 0.2 blood units per day. Long–term blood sparing is assumed to be the minimum value for excess oxygen transport capacity after HBOC treatment relative to the capacity value immediately before HBOC infusion. Since the pretreatment value is arbitrary in this analysis, it has been set equal to zero in this graph for convenience.
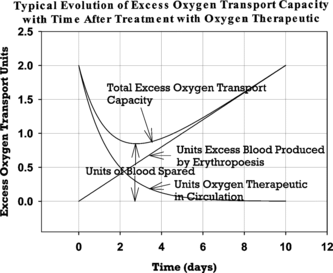
Figure 2 Comparison of data from clinical trials in surgical patients treated with HBOC solutions with mathematical model predictions. Model predictions for blood sparing are graphed as solid lines versus dose of HBOC for assumed half-lives and rates of erythropoesis enhancement noted to the right of each line. The first value in parentheses is the HBOC half-life in days. The second value is the rate of erythropoesis enhancement in blood units per day. Clinical data are plotted from published studies as noted in the text. Data from Biopure Corporation are plotted as dosing ranges with the data points placed in the range midpoint. The number of patients included in each trial data set is denoted by n.
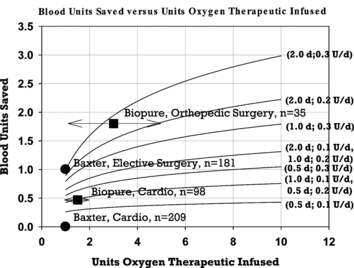
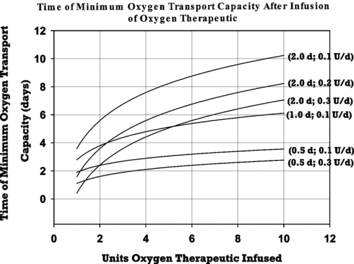
Figure 3 Variation in time after HBOC treatment at which total blood hemoglobin concentration attains a minimum value. Minimum points are plotted as a function of HBOC dose for HBOC half-lives and rates of erythropoesis enhancement noted at the right of each line. The notation for HBOC half-life and erythropoesis enhancement is as described in the caption of Figure 2.