Figures & data
Figure 1 Schematics of the model system. (A) Perfusion loop, consisting of channeled biorubber scaffolds (7), perfusion cartridges (4), two debubbling syringes (5, 6), a multi-channel peristaltic pump (1), a gas exchanger (2), and the reservoir bag (3). (B) Modes of oxygen transport in the channeled construct perfused with culture medium include convection through the channel lumen and diffusion into the tissue space surrounding each channel. In unsupplemented culture medium (left), oxygen dissolved in the aqueous phase during gas exchange in the external loop is transported into the tissue phase and consumed by the cells. In culture medium supplemented with perfluorocarbon (PFC) emulsion (right), oxygen is replenished within the tissue construct by the release from PFC particles, a process governed by Henry's law. (C) SEM showing parallel channels and porous structure of scaffold (100X, scale bar is 500 µm). (D) Cross section of a single channel showing key modeling parameters (Rc = channel radius, Rt = tissue radius) and cylindrical geometry of channel (z-axis = axial coordinate, r-axis = radial coordinate).
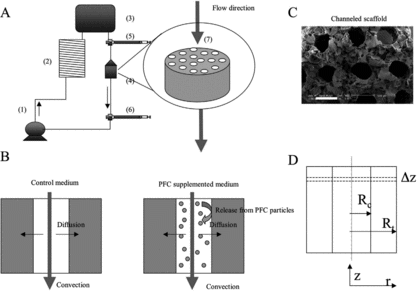
Table 1. Metabolic, contractile and biochemical properties of constructs cultivated with PFC emulsion and pure culture medium. * indicates significantly different than control medium by t-test, p < 0.05 considered significant. The values shown are Average±SE
Figure 2 Oxygen concentration profiles generated by FEMLAB for experimentally derived parameters. Oxygen profile is shown for half of a channel width for (A) unsupplemented culture medium and (B) PFC-supplemented culture medium (5.4% v/v).
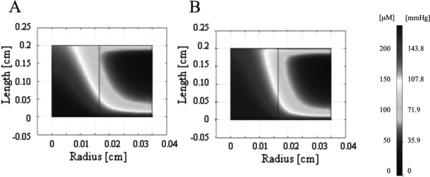
Figure 3 Log plot showing the oxygen concentration as a function of distance from the inlet at a position 100 µm away from the lumen. For a 330 µm channel diameter and 370 µm wall-to-wall spacing, at the highest PFC concentration of 6.4% v/v, and at a physiological cell density of 1 × 108 cells/cm3, the oxygen concentration drops several orders of magnitude along the length of the construct leading to sub-physiological oxygen levels throughout most of the tissue space.
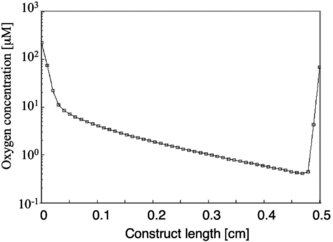
Figure 4 Oxygen concentration profile in the channel array. A 100 µm channel diameter and 100 µm wall-to-wall spacing was investigated in the model as an optimization to the in vitro parameters. The flow velocity was also increased in the range of 0.049 cm/s to 0.135 cm/s. A remarkable improvement in oxygen concentration is seen between (A) 0% PFC and (B) 6.4% PFC conditions.
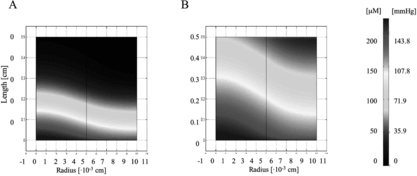
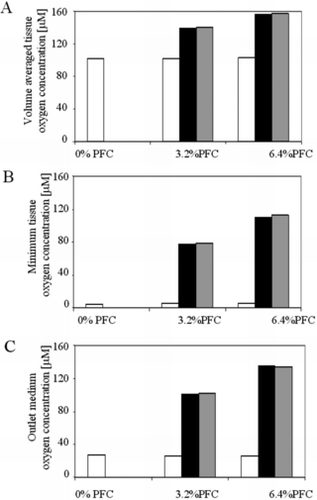
Figure 5 Overall effect of PFC emulsion on oxygen concentration in the cardiac constructs. (A) Volume average oxygen concentration, (B) minimum oxygen concentration, and (C) mixing cup outlet concentration of oxygen in medium are shown as functions of PFC fraction (0, 3.2%, 6.4%). The contribution of PFC to the effective diffusivity (white bars) is compared to the contribution of PFC to the convective transport term (black bars), and the combination of both (grey bars). As shown, the effect of the PFC is dominated by its contribution to convective transport of oxygen.