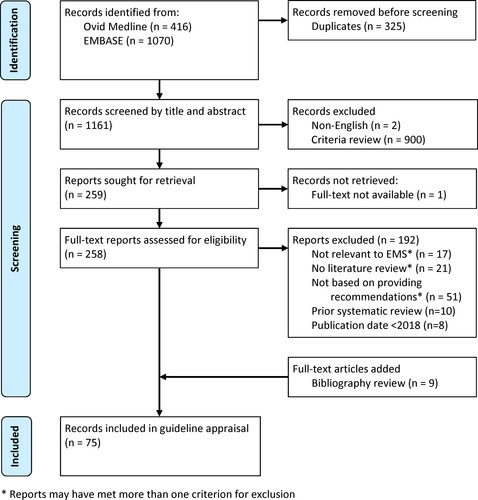
Figures & data
Table 1. Criteria for clinical practice guidelines assessment.
Table 2. Appraisal of guidelines for research and evaluation (AGREE) II assessment.
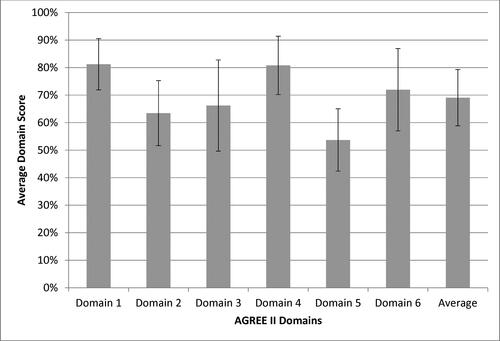
Figure 2. Average scores across AGREE II domains (average of three appraisers ± standard deviation).
Domain 1 – Scope and Purpose (description of objectives, questions, and population)
Domain 2 – Stakeholder Involvement (description of input gained from stakeholders including target population)
Domain 3 – Rigor of Development (description of methodology of evidence evaluation and development of recommendations)
Domain 4 – Clarity of Presentation (clarity of recommendations including options for management)
Domain 5 – Applicability (description of implementation and evaluation)
Domain 6 – Editorial Independence (description of potential conflicts from funding body or guideline development group members)