Figures & data
Figure 2. a– Group I: Dilatation and haemorrhage in the vessels of the pulp, an increase in collagen fibres, degeneration in odontoblast cells, and a widening of the periodontal membrane and alveolar spaces were observed (H-E staining Bar 100 µm); b – Group II: Haemorrhage in the small vessels of the pulp, hyperplasia and degeneration in odontoblastic cells and dilatation and haemorrhage in the vessels of the periodontal membrane were observed (H-E staining Bar 100 µm); c – Group III: Proliferation in the connective tissue cells in the pulp, regular alignment in collagen fibres and increased odontoblastic activity were observed (H-E staining Bar 100 µm); d -- Group V: Increased dilatation and haemorrhage in the vessls of the pulp, an increase in fibrous tissue and leukocyte infiltrations and loss of tissue with necrotic tissue and hyalinisation in the periodontal membrane were observed (H-E staining Bar 100 µm); e – Group VI: Dilatation in the pulp vessels and inflammatory cell infiltrations surrounding the vessel and degenerative changes in odontoblastic cells were observed (H-E staining Bar 50 µm); f -- Regular alignment was observed in the odontoblast cells at the border of the dentin and the pulp (arrow) and no change in the fibrous, vascular structure or cellular alignment in the pulp (H-E staining Bar 50 µm).
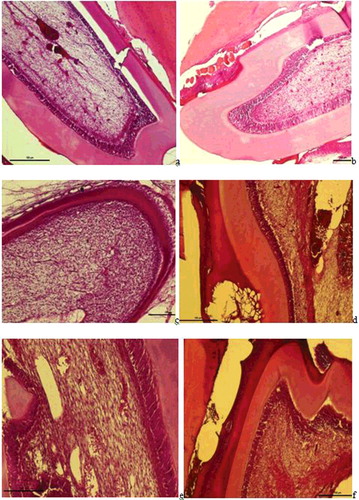
Figure 3. a– Group I: VEGF positive expression observed in dlated vessels and inflammatory cell infiltrations in the pulp (VEGF immune staining 100 µm); b – Group II: VEGF positive reaction in the vessel wall in the pulp (red arrow), reduced and weak VEGF expression in inflammatory cells (VEGF immune staining 100 µm); c – Group III: VEGF positive expression in the vessels close to odontoblastic cells and in vessels in the cement-periodontal space (red arrow) and neo-angiogenesis was seen to have been induced (VEGF immune staining 100 µm); d – Group V: VEGF positive expression in the endothelial cells of the expanded vessel wall in the pulp, in the small vessels between degenerative odontoblast cells and in inflammatory cells (VEGF immune staining 50 µm); e – Group VI: VEGF positive expression in the vessels of the periodontal ligaments and pulp (VEGF immune staining 100 µm), f – Group VII: VEGF positive reaction in the small blood vessels of the pulp and in some inflammatory cells (VEGF immune staining 50 µm).
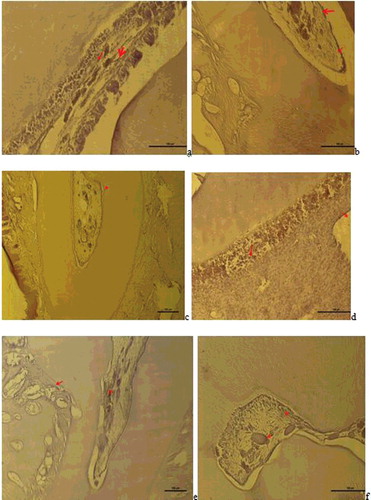
Figure 4. a – Group I: Vimentin expression was observed in degenerative odntoblast cells, fibroblast cells and impaired fibrous structures in the pulp (Vimentin immune staining 50 µm); b – Group II: Vimentin was positive in the degenerative change in the fibres of the periodontal membrane and weak Vimentin expression was observed in scattered fibre distribution in the pulp (Vimentin immune staining100 µm); c – Group III: Vimentin positive expression was observed in odontoblast cells and fibroblast cells and a positive Vimentin reaction was observed in the regular fibre organisation in the pulp (Vimentin immune staining50 µm); d – Group V: Degenerative change was observed in the odontoblast cells of the pulp and positive Vimentin reaction in the impaired fibrous structure around the dilated vessel and in areas of hyalinisation (Vimentin immune staining50 µm); e – Group VI: A positive Vimentin reaction was observed in odontoblasts and in the fibrous structure around the vessel (Vimentin immune staining50 µm); f – Group VII: A positive Vimentin reaction was observed in a diffuse form within the pulp fibroblasts with regular odontoblastic alignment between the dentin and pulp (Vimentin immune staining 50 µm).
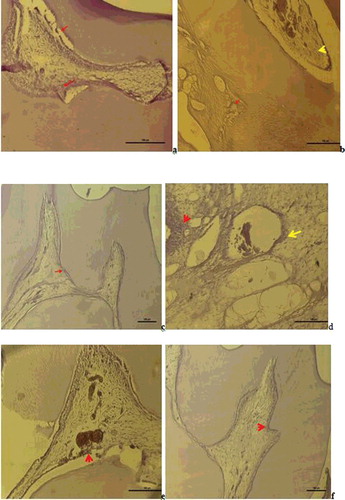
Table 1. The statistical results of odontoblast cell degeneration scores in groups. The values represent mean ± SD. A statistically significant difference was determined between the control group and Groups I, II, V and VI in respect of odontoblast degeneration as a result of ELF-EMF exposure.
Table 2. The statistical results of Inflammatory cell infiltration scores in groups. The values represent mean ± SD. A statistically significant difference was determined between the control group and groups I, II, V and VI in respect of inflammatory cell infiltration as a result of ELF-EMF exposure.
Table 3. The statistical results of dilatation and hemorrhage in blood vessels scores in groups. The values represent mean ± SD. While no statistically significant difference was determined between the control group and Group II in respect of vasodilation in the pulp blood vessels and haemorrhage as a result of ELF-EMF exposure. However, a statistically significant difference was determined between the control group and Groups I, V and VI.