Figures & data
Figure 1. 3H-DFP incorporation into rat plasma albumin after preincubation with pesticide-oxons. Rat plasma was incubated with pesticide-oxons or solvent at 20 min thymus tissue AChE IC30 concentrations. Reactive hydrolase groups were then adducted by incubation with 3H-DFP for 1 h at 37°C. (A) Ten micrograms of radiolabelled plasma was loaded onto glass microfibre filters, washed, and the incorporation of 3H-DFP determined. Results are presented as the mean±standard error from at least 16 independent experiments with each pesticide. Results were significantly different from controls with chlorfenvinphos (oxon), chlorpyrifos-oxon, and diazinon-oxon (*** p <0.001). (B) Twenty micrograms of radiolabelled plasma was resolved by SDS-PAGE, and then transferred to a polyvinylidene difluoride membrane. Proteins were stained with Coomassie brilliant blue (left panel) or autoradiographed (right panel). The positions of protein molecular weight markers are shown in the first two lanes of the gel and blot. A single radiolabelled protein band was evident (marked with an arrowhead) which superimposed with the leading edge of the albumin protein band detected from protein staining. Incubation with the pesticide-oxons of chlorfenvinphos, chlorpyrifos, and diazinon significantly reduced 3H-DFP incorporation in to this radiolabelled protein.
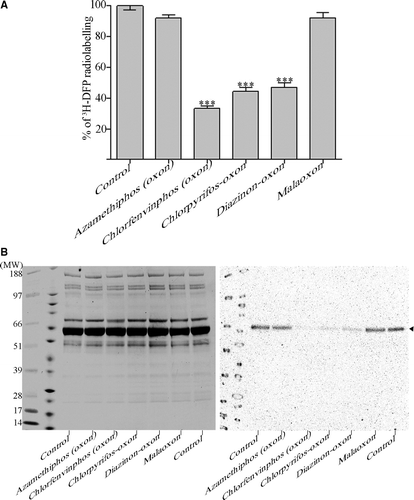
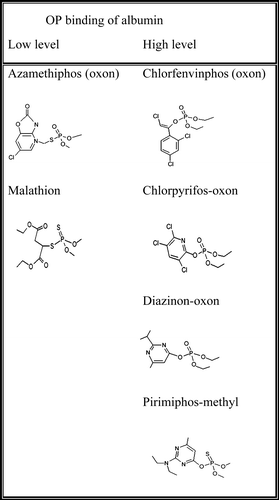
Table I. Stiochiometry of 3H-DFP incorporation into rat plasma albumin. Rat plasma albumin (2.3 µM) was incubated with solvent or pesticide-oxons at 20 min AChE IC30 concentrations, and then radiolabelled with 5.5, 10 or 19 µM 3H-DFP. Both the increase in albumin radiolabelling from increasing 3H-DFP concentration, and the level of pesticide binding relative to controls was determined by quantifying the level of radiolabelled albumin retained on glass microfibre filters.
Figure 2. Time course of 3H-DFP incorporation into rat plasma albumin. Rat plasma (300 µg) was reacted at 37°C with 19 µM 3H-DFP (19 nmoles) in a buffer of 10 mM Tris/HCl pH 8.0 containing 1 mM EDTA, 5 mM DTT, 5% glycerol (1000 µl final volume). At intervals of 1, 2, 4, 24, 48, 72, and 96 h, replicate 50 µl samples were removed and the level of 3H-DFP incorporated into albumin quantified by counting the radiolabelled albumin retained on glass microfibre filters. To counter 3H-DFP hydrolysis during the time course, the reaction mixture was supplemented with 7.2 nmoles of 3H-DFP after 24 h, 4.8 nmoles of 3H-DFP after 48 h, and 2.4 nmoles of 3H-DFP after 72 h. Data points are mean±standard deviation from four independent experiments.
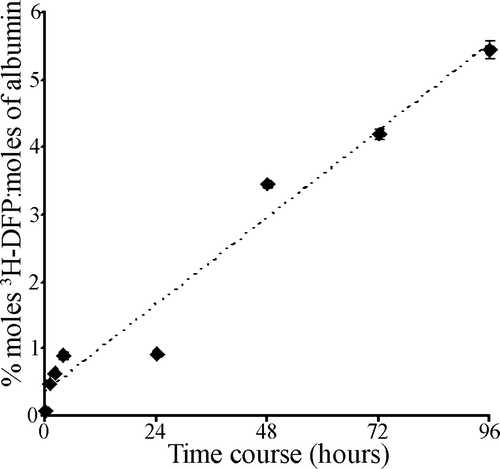
Table II. Stiochiometry of 3H-DFP incorporation into rat and human albumins. Rat or human albumin (1–2 µM) was incubated with solvent or pesticide-oxons at 20 min AChE IC30 concentrations, and then radiolabelled with 19 µM 3H-DFP. The level of albumin radiolabelling was quantified by counting radiolabelled albumin retained on glass microfibre filters. For rat plasma albumin, n=16, and for all other data points n=4–6 experiments.
Figure 3. Comparison of pesticide binding of albumin with inhibition of AChE. Rat plasma (203 µg ml−1) was incubated with pesticide or solvent for 20 min at room temperature over the pesticide concentration ranges shown. Proteins were then radiolabelled with 17 µM 3H-DFP for 1 h at 37°C. The radiolabel incorporated into albumin was quantified by counting radiolabelled albumin retained on glass microfibre filters. The corresponding inhibition of AChE at each concentration was quantified using the Ellman assay, with the results presented graphically. Radiolabelling of albumin was also visualised after SDS-PAGE and autoradiography and is shown in the lower panels. (A) Chlorfenvinphos (oxon), (B) chlorpyrifos-oxon, (C) diazinon-oxon.
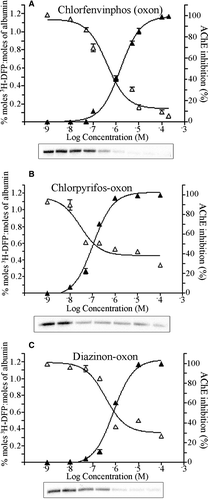
Figure 4. Quantitation of dimethoxy- and diethoxy-pesticide binding of albumin in vivo. Rats were treated with solvent (controls) or dosed with 25% of the LD50 of pirimiphos-methyl (A), or 25% of the LD50 of chlorfenvinphos (oxon) (B). After radiolabelling with 3H-DFP the incorporation of radioactivity into albumin was quantified by counting the radiolabelled albumin retained on glass microfibre filters. Results are presented as the mean±standard error from at least four independent experiments from each rat. Results were significantly different from controls at 1, 3, and 7 days after dosing (***p <0.001) for both pesticides. Radiolabelled albumin was also resolved by SDS-PAGE, and an example of the level of radioactivity incorporated into albumin for each dose condition displayed in the lower panels. The average level of erythrocyte AChE inhibition for each dosing condition is also included.
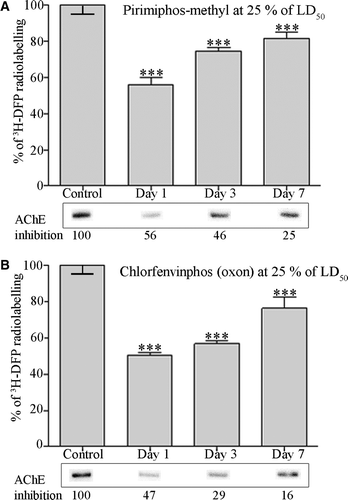
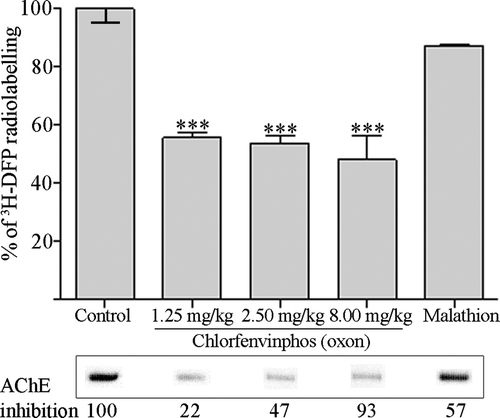
Figure 5. Albumin is maximally bound across a range of chlorfenvinphos (oxon) doses in vivo, but is not significantly bound by malathion in vivo. Rats were treated with solvent (controls) or dosed with 12.5%, 25%, and 80% of the LD50 of chlorfenvinphos (oxon), or approximately 25% of the LD50 of malathion. Twenty-four hours after dosing plasma albumin was radiolabelled with 3H-DFP, and the incorporation of radioactivity into albumin quantified by counting the radiolabelled albumin retained on glass microfibre filters. Results are presented as the mean±standard error from at least four independent experiments from each rat. Results significantly different from controls are marked (***p <0.001). Radiolabelled albumin was also visualised by autoradiography after SDS-PAGE, and an example of the level of radioactivity incorporated into albumin shown in the lower panels. The average level of erythrocyte AChE inhibition for each dosing condition is also included.