Figures & data
Table 1. Inputs into the cost-effectiveness model: base case.
Table 2. Base case analysis: costs, outcomes, and cost-effectiveness of denosumab vs zoledronic acid in patients with prostate cancer, breast cancer, or other solid tumors.
Table 3. Scenario analyses: effect of varying discount rate, time horizon, SRE rate, discontinuation of treatment, and asymptomatic SREs on the cost-effectiveness of denosumab vs zoledronic acid.
Figure 1. Tornado diagram for (a) prostate cancer, (b) breast cancer, and (c) other solid tumors. CZK: Czech Republic Koruna; ICER: incremental cost-effectiveness ratio; IV: intravenous; ONJ: osteonecrosis of the jaw; QALY: quality-adjusted life-year; SC: subcutaneous; SRE: skeletal-related event.
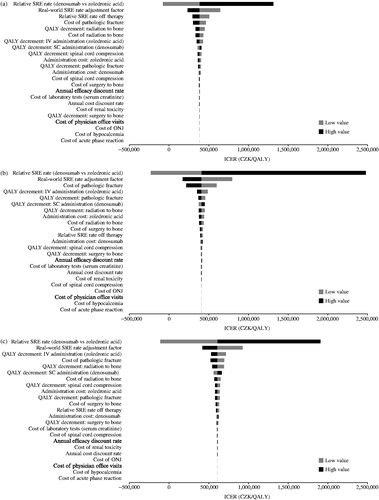
Figure 2. Cost-effectiveness acceptability curves for (a) prostate cancer, (b) breast cancer, and (c) other solid tumors. CZK: Czech Republic Koruna; QALY: quality-adjusted life-year.
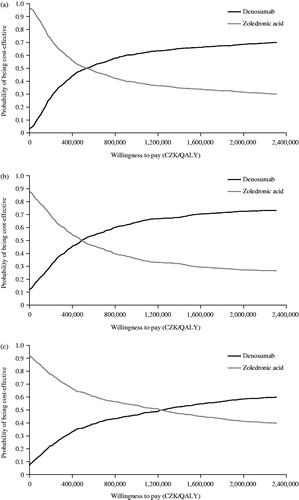
Table A1. Prostate cancer probabilistic inputs.
Table A2. Breast cancer probabilistic inputs.
Table A3. Other solid tumors probabilistic inputs.

