Figures & data
Table 1. Base-case model inputs and ranges used in one-way sensitivity analysis.
Table 2. Drug costs per patient per year in rivaroxaban and placebo cohorts.
Table 3. Clinical event costs per patient per year in rivaroxaban and placebo cohorts.
Figure 1. Total healthcare cost difference per patient per year between rivaroxaban- and placebo-treated patients.
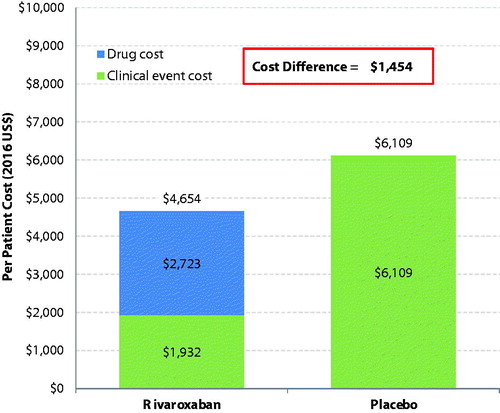
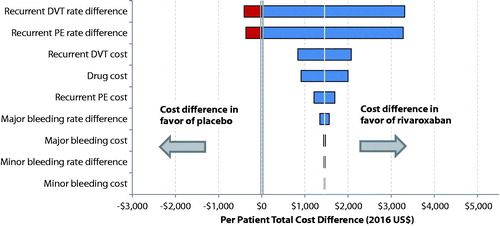
Figure 2. Total healthcare cost difference per patient per year between rivaroxaban- and placebo-treated patients estimated in one-way sensitivity analysis. (1) Unit costs associated with drug, recurrent DVT, recurrent PE, major bleeding, or clinically relevant non-major bleeding varied by having its base unit cost decreased or increased by 20%. (2) The variation of a clinical event rate (i.e. recurrent DVT, recurrent PE, major bleeding, or clinically relevant non-major bleeding) was assessed based on the corresponding 95% confidence intervals (CIs) of rate differences between cohorts that were reported in the EINSTEIN-EXT study (Wells et al.Citation17).