Figures & data
Figure 1. Markov model of ulcerative colitis treatment progression. Abbreviation. 5-ASA, 5-aminosalicylic acid.
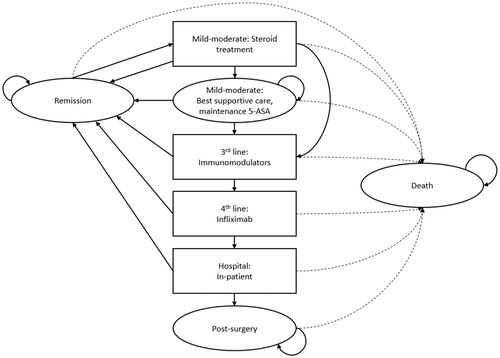
Table 1. Network meta-analysis results.
Table 2. Transition matrix with probabilities shared between interventions.
Table 3. Pharmaceutical unit costs.
Table 4. Unit costs of additional healthcare.
Table 5. Utility inputs.
Table 6. Budesonide MMX and comparator cost-effectiveness.
Table 7. Deterministic sensitivity analysis inputs and outcomes.
Figure 2. Cost-effectiveness plane for budesonide MMX versus comparator. Abbreviation. QALYs, quality-adjusted life years. Incremental costs displayed in 2016 Euros.
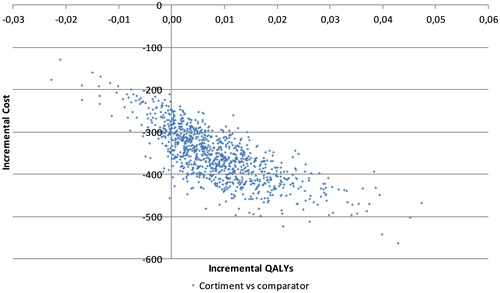
Table 8. Scenario analysis with alternative comparator combinations.
