Figures & data
Table 1. Key model parameters, assumptions, and cost variables.
Figure 1. Model structure showing (a) the treatment sequence for the base-case scenario, (b) patient progress in the model, and (c) the mechanism for estimating costs, mortality, and quality-of-life based on disease severity, measured by the HAQ-DI. Abbreviations. BID, twice daily; HAQ-DI, Health Assessment Questionnaire-Disability Index; MTX, methotrexate; Q2W, every 2 weeks; SAE; serious adverse event.
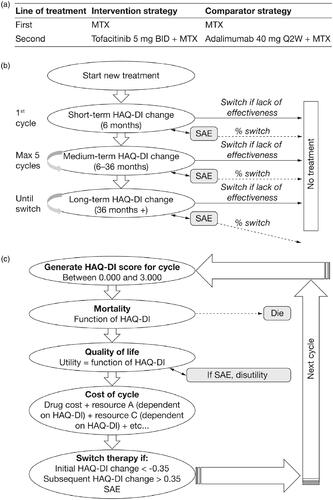
Table 2. Base-case cost-effectiveness results for tofacitinib plus MTX vs adalimumab plus MTX as second-line therapy in patients with RA.
Figure 2. Tornado diagram representing the net benefit in one-way sensitivity analyses with changing baseline parameters. The width of the bars represents the range of results when variables were changed. aHAQ-DI – utility was calculated using the following parameters (lower boundary, upper boundary): constant term (0.800, 0.758), linear term (−0.247, −0.259), quadratic term (−0.031, −0.045), age covariate (0.002, 0.001), gender covariate (0.042, 0.020), RA duration covariate (0.002, 0.000). bHAQ-DI – cost was calculated using the following parameters (lower boundary, upper boundary): Class I (NT$50,568, NT$33,712), Class II (NT$65,453, NT$43,635), Class III (NT$120,019, NT$80,013), Class IV (NT$75,197, NT$50,131). Abbreviations. HAQ-DI, Health Assessment Questionnaire-Disability Index; LOE, loss of effect; LT, long-term; MT, medium-term; N/A, not applicable; NT$, New Taiwan Dollars; QALY, quality-adjusted life-year; RA, rheumatoid arthritis; SAE, serious adverse event; ST, short-term.
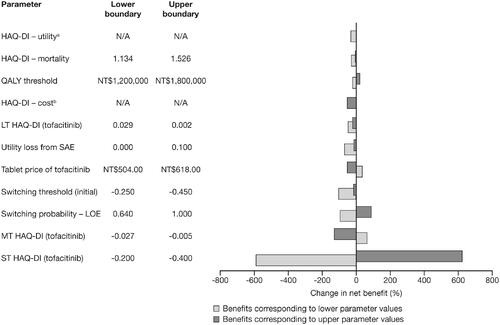
Table 3. One-way sensitivity analysis of cost-effectiveness results for tofacitinib in combination with methotrexate vs adalimumab plus methotrexate as second-line therapy in rheumatoid arthritis.
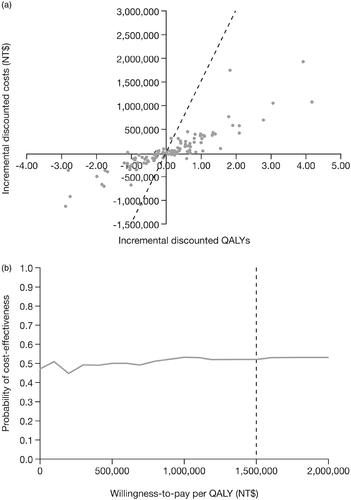
Figure 3. Cost-effectiveness planes for (a) the probabilistic sensitivity analyses, and (b) cost-effectiveness acceptability curve for tofacitinib plus MTX vs adalimumab plus MTX as second-line therapy of patients with RA. The dotted line represents the cost-effectiveness threshold per QALY gained. Abbreviations. MTX, methotrexate; NT$, New Taiwan Dollars; QALY, quality-adjusted life-year; RA, rheumatoid arthritis.
Data availability statement
Upon request, and subject to certain criteria, conditions, and exceptions (see https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information), Pfizer will provide access to individual de-identified participant data from Pfizer-sponsored global interventional clinical studies conducted for medicines, vaccines, and medical devices (1) for indications that have been approved in the US and/or EU or (2) in programs that have been terminated (i.e. development for all indications has been discontinued). Pfizer will also consider requests for the protocol, data dictionary, and statistical analysis plan. Data may be requested from Pfizer trials 24 months after study completion. The de-identified participant data will be made available to researchers whose proposals meet the research criteria and other conditions, and for which an exception does not apply, via a secure portal. To gain access, data requestors must enter into a data access agreement with Pfizer.

