Figures & data
Figure 1. Study design. Abbreviations: EBC, Early breast cancer; SEER, Surveillance Epidemiology and End Results; HCRU, Health-care resource utilization.
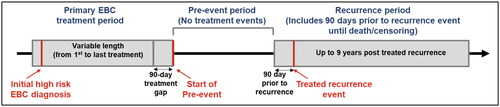
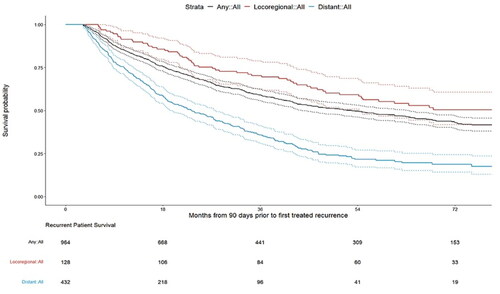
Figure 2. Patient attrition and final sample size. Abbreviations: AJCC, American Joint Committee on Cancer; ESRD, end-stage renal disease; HR+/HER2−, hormone receptor positive/human epidermal growth factor receptor 2; ID, SEER, Surveillance, Epidemiology, and End Results Program of the National Cancer Institute. Notes: *Complete record defined as non-missing year of diagnosis, tumor site, AJCC stage, breast subtype, and AJCC N from SEER data. †Sequence number for first tumor is 0 when the breast cancer tumor is the patient’s first and only tumor and 1 when the breast cancer tumor is the patient’s first of multiple tumors. ‡High risk patients are defined as those who had 4–90 regional node-positive lymph nodes, or 1–3 regional node-positive lymph nodes (SEER variable: Regional_nodes_positive_1988) and either tumor grade 3 (SEER variable: Grade) or tumor size greater than or equal to 5 cm (SEER variable: CS_tumor_size_2004_2015).
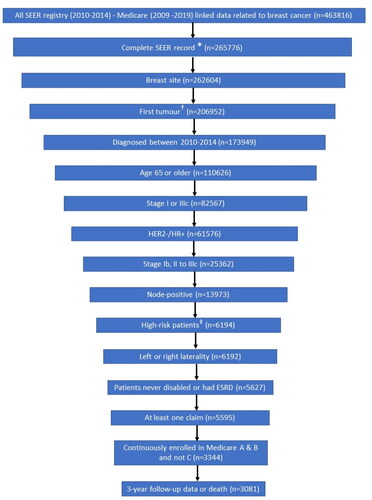
Table 1. Patient demographics and clinical characteristics – overall population and Part D.
Table 2. Comparison of characteristics of treated recurrence in the primary analysis set and Part D subgroup.
Figure 3. Extra cost of recurrence for all recurrence patients by month after recurrence over the first 6 years of follow-up period.
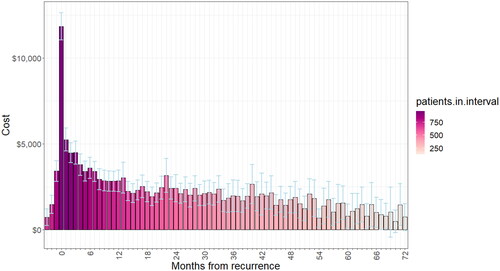
Table 3. Estimated mean extra cost of recurrence, by period after recurrence.
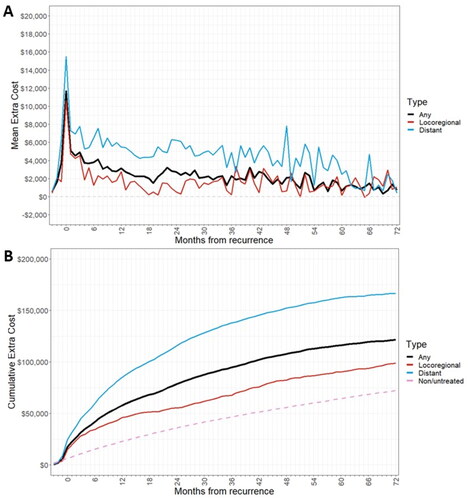
Figure 4. (a) Extra cost of recurrence by month after recurrence, by recurrence type (b) cumulative treatment costs post-recurrence, by recurrence type.
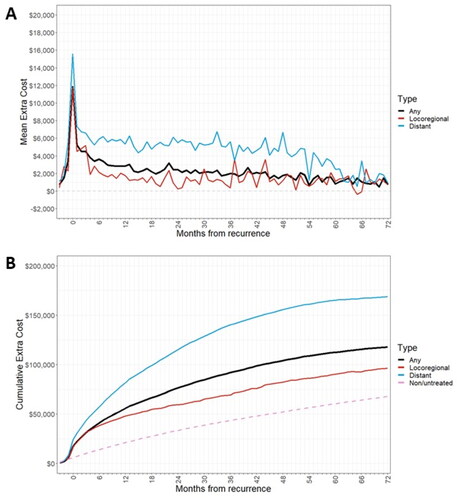
Table 4. Cumulative treated recurrence costs by recurrence type in primary analysis set and Part D subgroup.
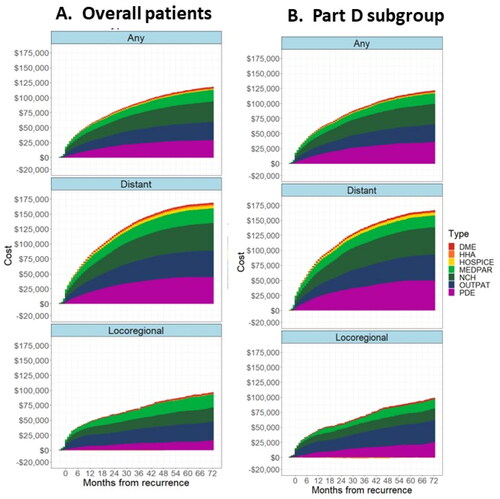
Figure 5. Cumulative treatment costs by type of service (a) overall population and (b) Part D subgroup. Abbreviations: DME, Durable Medical Equipment; HHA, Home Health Agency; MEDPAR, Medicare Part A Inpatient; NCH, National Claims History (Medicare Part B Non-institutional); OUTPAT, Medicare Part B Institutional; PDE, Part D Drug Event. Notes: Primary population is the full primary analytical population. Part D subgroup is the analysis of the subset of patients who had continuous Part D enrollment from 1-month prior to their diagnosis of breast cancer until their exit date.
Supplemental Material
Download MS Word (176.8 KB)Data availability statement
The data that support the findings of this study are available from the SEER-Medicare linked database (https://healthcaredelivery.cancer.gov/seermedicare/), but restrictions apply to the availability of these data, which were used under permission for the current study, and so are not publicly available. Data are available from SEER-Medicare upon reasonable request and with permission.