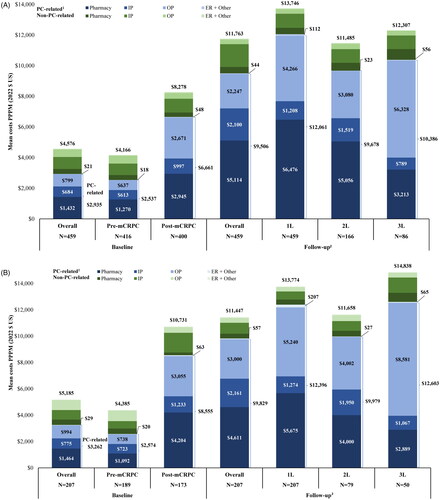
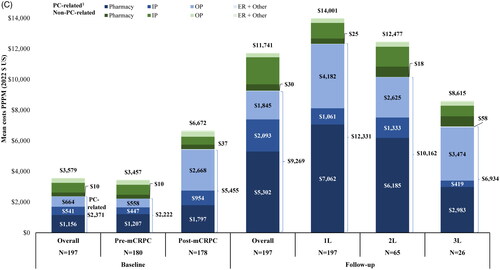
Figures & data
Figure 1. Study design scheme.
1L: first-line; HRU: Healthcare resource utilization; LOT: line of therapy; mCRPC: metastatic castration-resistant prostate cancer.
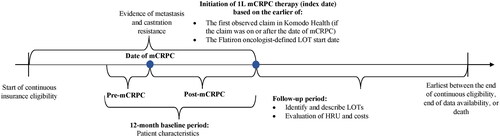
Figure 2. Patient identification flowchart.
1L: first-line; ARSIs: androgen receptor signaling inhibitors; EMR: electronic medical records; LOT: line of therapy; mCRPC: metastatic castration-resistant prostate cancer; PARP: poly ADP-ribose polymerase; PC: prostate cancer.
Note:
1. Medications considered for 1L mCRPC therapy were: ARSIs (i.e. apalutamide, darolutamide, enzalutamide, abiraterone acetate), chemotherapy (i.e. cabazitaxel, carboplatin, cisplatin, docetaxel, etoposide, mitoxantrone), PARP inhibitors (i.e. niraparib, olaparib, rucaparib, talazoparib), immunotherapy (i.e. sipuleucel-T, pembrolizumab), estrogens (i.e. estramustine phosphate, diethystillbestrol, polyestradiol phosphate), radiopharmaceuticals (i.e. radium-223, lutetium-177-PSMA-617).
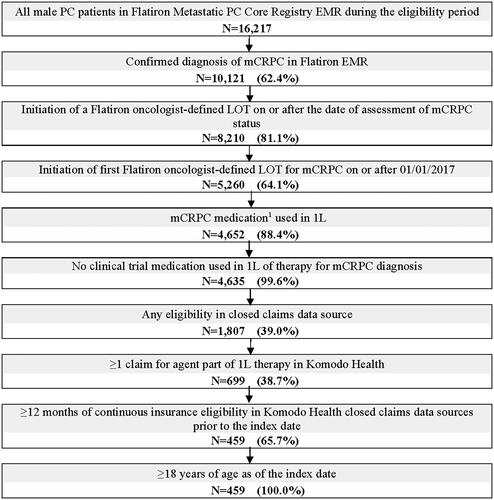
Table 1. Baseline characteristics.
Table 2. Baseline and follow-up HRU1 PPPM.
Figure 4. Regimens used as Flatiron oncologist-defined 1 L, 2 L, and 3 L mCRPC therapyCitation1,Citation2.
1L: first-line; 2L: second-line; 3L: third-line; ARSI: androgen receptor signaling inhibitor; Chemo: chemotherapy; mCRPC: metastatic castration-resistant prostate cancer.
Notes:
1. All individual agents reported were used as monotherapy in Flatiron oncologist-defined 1L, 2L, or 3L mCRPC therapy.
2. Medications considered for other monotherapy were: ARSIs (i.e. darolutamide), chemotherapy (i.e. cisplatin, mitoxantrone), PARP inhibitors (i.e. olaparib), and immunotherapy (i.e. pembrolizumab).
3. Among 164 patients treated with abiraterone acetate monotherapy as 1L, 54 (32.9%) had a claim for generic abiraterone acetate while 110 (67.1%) had a claim for branded abiraterone acetate (i.e. Zytiga [108] or Yonsa [2]) as their first claim on or after the date of mCRPC.
![Figure 4. Regimens used as Flatiron oncologist-defined 1 L, 2 L, and 3 L mCRPC therapyCitation1,Citation2.1L: first-line; 2L: second-line; 3L: third-line; ARSI: androgen receptor signaling inhibitor; Chemo: chemotherapy; mCRPC: metastatic castration-resistant prostate cancer.Notes:1. All individual agents reported were used as monotherapy in Flatiron oncologist-defined 1L, 2L, or 3L mCRPC therapy.2. Medications considered for other monotherapy were: ARSIs (i.e. darolutamide), chemotherapy (i.e. cisplatin, mitoxantrone), PARP inhibitors (i.e. olaparib), and immunotherapy (i.e. pembrolizumab).3. Among 164 patients treated with abiraterone acetate monotherapy as 1L, 54 (32.9%) had a claim for generic abiraterone acetate while 110 (67.1%) had a claim for branded abiraterone acetate (i.e. Zytiga [108] or Yonsa [2]) as their first claim on or after the date of mCRPC.](/cms/asset/dfd9058b-f7ca-4e52-94f1-e17a49376d31/ijme_a_2303890_f0004_c.jpg)
Supplemental Material
Download MS Word (48.5 KB)Data availability statement
Data that support the findings of this study were used under license from Flatiron Health, Inc. and Komodo Health Solutions. Restrictions apply to the availability of these data, which are not publicly available and cannot be shared. The data are available through request made directly to the data vendor, subject to the data vendor’s requirements for data access.