Figures & data
Figure 1. Study design scheme. ADT: androgen deprivation therapy; HRU: healthcare resource utilization; LOT: line of therapy; mCSPC: metastatic castration-sensitive prostate cancer.
Figure 2. Identification of the study population. ADT: androgen deprivation therapy; ARSIs: androgen receptor signaling inhibitors; EMR: electronic medical records; LOT: line of therapy; mCRPC: metastatic castration-resistant prostate cancer; mCSPC: metastatic castration-sensitive prostate cancer; PARP: poly ADP-ribose polymerase; PC: prostate cancer.
aMedications considered as advanced treatment for mCSPC therapy were: ARSIs (i.e., apalutamide, darolutamide, enzalutamide, abiraterone acetate), chemotherapy (i.e., cabazitaxel, carboplatin, cisplatin, docetaxel, etoposide, mitoxantrone), PARP inhibitors (i.e., niraparib, olaparib, rucaparib, talazoparib), immunotherapy (i.e., sipuleucel-T, pembrolizumab), estrogens (i.e., estramustine phosphate, diethystilbestrol, polyestradiol phosphate), radiopharmaceuticals (i.e., radium-223, lutetium-177-PSMA-617).
bRecords for medications used as advanced treatment for mCSPC were evaluated in the Flatiron oncologist-defined LOT tables, as well as medication orders, administrations, and oral tables.
cPatients with clinical trial medication were excluded.
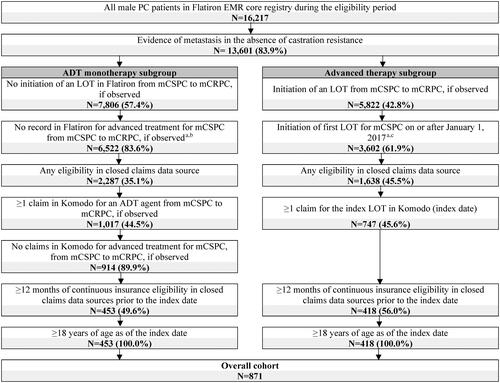
Table 1. Baseline demographic and clinical characteristics.
Table 2. Baseline and follow-up HRU PPPM.
Figure 3. Baseline and follow-up costs PPPM. ARSI: androgen receptor signaling inhibitor; ER: emergency room; HRU: healthcare resource utilization; ICD-10-CM: International Classification of Diseases 10th Revision Clinical Modification; IP: inpatient; LHRH: luteinizing hormone-releasing hormone; OP: outpatient; mCSPC: metastatic castration-sensitive prostate cancer; PARP: poly ADP-ribose polymerase; PC: prostate cancer; PPPM: per-patient-per-month; US: United States.
Notes:
aPC-related HRU and costs were identified with the ICD-10-CM code C61 and procedure codes for LHRH or of the following guideline-recommended therapies for mCSPC: ARSIs, chemotherapy, PARP inhibitors, immunotherapy, estrogens, and radiopharmaceuticals.
bThe mCSPC pre-treatment period was defined as the portion of the 12-month baseline period that occurred on and after evidence of metastasis but prior to the initiation of therapy.
cThe follow-up period was defined as the time from the index date until the earliest of i) evidence of castration resistance, ii) end of continuous insurance eligibility, iii) end of data availability, or iv) death (if available).
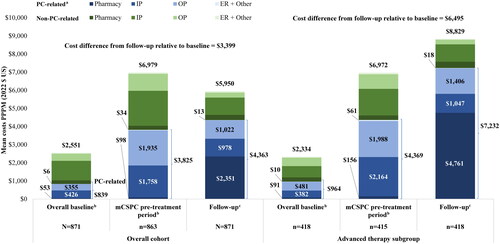
Table 3. Baseline and index treatment patterns.
Supplemental Material
Download MS Word (16.3 KB)Data availability statement
Data that support the findings of this study were used under license from Flatiron Health, Inc. and Komodo Health Solutions. Restrictions apply to the availability of these data, which are not publicly available and cannot be shared. The data are available through request made directly to the data vendor, subject to the data vendor’s requirements for data access.
