Figures & data
Table 1. Post-approval indications.
Table 2. Drugs, indications, and industry-funded clinical trials by approval-year cohort.
Figure 1. Post-approval indications per drug. Source: Authors’ calculations of Drugs@FDA data. Notes. Post-approval indications have approval dates after US drug approval date.
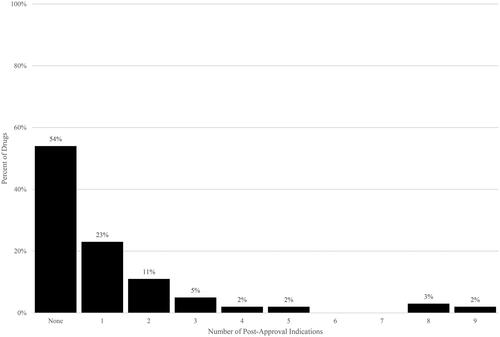
Figure 2. Post-approval industry-funded trial starts per drug. Source: Authors’ calculations of ClinicalTrials.gov data. Notes. Post-approval trials have start dates after US drug approval date.
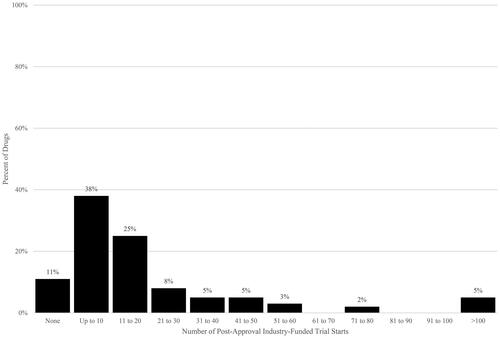
Figure 3. Trial enrollment in post-approval industry-funded trials. Source: Authors’ calculations of ClinicalTrials.gov data. Notes. Post-approval trials have start dates after US drug approval date.
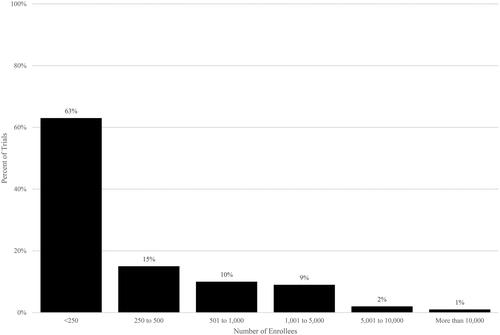
Table 3. Post-approval indications and industry-funded clinical trials by years from US drug approval (small molecules only).