Figures & data
Figure 1. Model schematic.
This schematic represents treatment with advanced therapies as 8-week induction and 44-week maintenance phases. Duration of induction and maintenance phases varies according to differing specifications for each advanced therapy in the model (). Patients classified as non-responders were switched to either of the alternative treatment scenarios (i) 5-biologics-basket (weighted average of infliximab, adalimumab, vedolizumab, ustekinumab, and golimumab) or (ii) no treatment. Non-responder frequency of visits was once per month. Response rate was stable until the end of the maintenance phase (formula applied: 1-clinical response rate). Patients in the responder population, continued the advanced therapies they received in the induction phase once per two months. Clinical response was reduced in the same proportion every month.
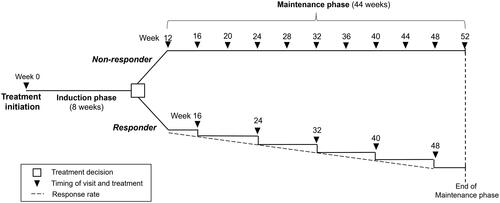
Table 1. Comparators in the model.
Table 2. Median efficacy rates with median 95% credible intervals.
Table 3. SAE rates.
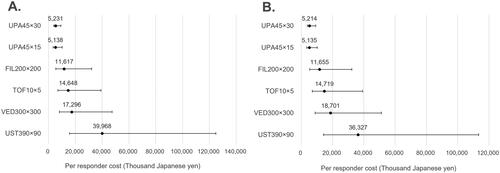
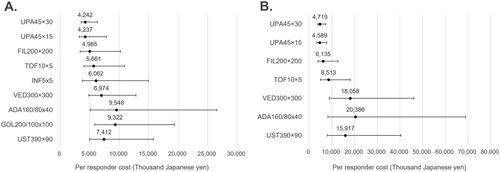
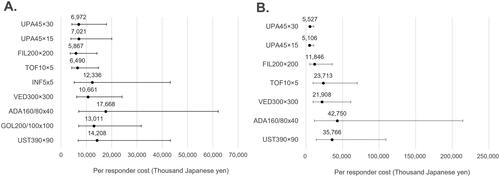
Figure 2. Cost per responder for clinical remission (5-biologic-basket for non-responders). (A) Biologic-naïve cost per clinical remission; (B) biologic-exposed cost per clinical remission; in figure, dots represent the median cost per responder and the whiskers represent the 95% CrI.
Supplemental Material
Download MS Word (153.6 KB)Data availability statement
The data supporting the findings of this study are available within the article and its online supplementary materials.