Figures & data
Table 1. Cost-effectiveness model design and settings.
Figure 1. AxSpA treatment-pathways applied in the model. AxSpA treatment-pathways applied in the model were defined in line with ASAS/EULAR and SmPC recommendations. In Line 2, TNFi were taken as a class, with efficacy data from an SLR/NMA and cost data based on individual TNFi list prices weighted by their estimated market shares in Scotland. ASAS, Assessment in Spondyloarthritis international Society; BSC, best supportive care; BKZ, bimekizumab treatment-pathway; b/ts, biologic/targeted synthetic; DMARD, disease modifying antirheumatic drug; EULAR, European League Against Rheumatism; IL, interleukin; IXE, ixekizumab treatment-pathway; nr-/r-axSpA, non-radiographic/radiographic axial spondyloarthritis; SEC, secukinumab treatment-pathway; SmPC, summary of product characteristics; TNFi, tumor necrosis factor-alpha inhibitor.

Figure 2. Model structure: decision tree for first year treatment-pathway. Patients with active axSpA start a treatment-pathway with a first b/tsDMARD (L1). At the end of the initial 16-week assessment period, their clinical response is evaluated: (a) BASDAI50 responders continue initial treatment L1; a proportion of L1 responders will however discontinue L1 between w17 and w52 (any cause discontinuation, assumed to occur at an annual rate of 11%). After L1 discontinuation, patients get a next b/tsDMARD treatment (L2) which is assumed to start with the Markov model as from year 2. (b) BASDAI50 non responders stop initial treatment L1 and start a next b/tsDMARD treatment (L2) for another 16-week assessment period (or 12 weeks if L2 is a TNFi) with clinical response evaluation: BASDAI50 responders start L2 maintenance treatment, subject to a 11% annual discontinuation rate for any cause between w28 and w52, after which they receive BSC. BASDAI50 non-responders stop L2 treatment and receive BSC from w28 onwards until the end of the first year. The treatment-pathways modeled for one year determine the initial health states distribution for the Markov model. No discontinuation was assumed to occur during an assessment period (week 1–16 for L1, and week 17–28 for L2). axSpA, axial spondyloarthritis; BSC, best supportive care; L1/2, line 1/2; resp., response; Tx, treatment.
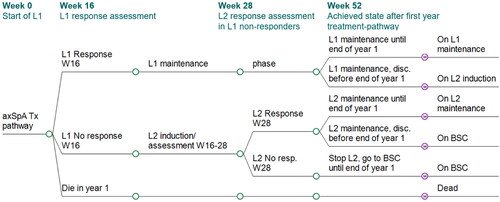
Figure 3. Model structure: Markov model as from second year of treatment until death. Initial distribution determined by outcome of decision tree. Three-month Markov cycles until end of horizon (or death). BSC, best supportive care; disc, discontinuation; L1/2, biologic line 1/2; resp, responder.
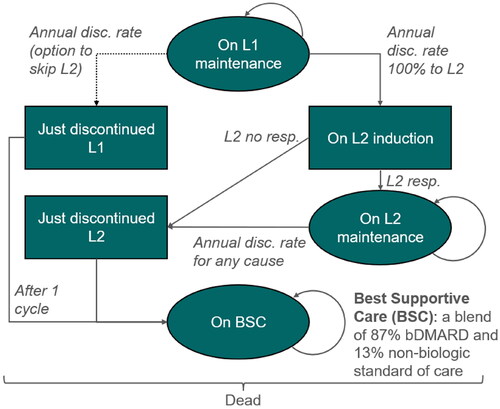
Table 2. Efficacy and safety data inputs.
Table 3. Healthcare resource use and cost inputs.
Figure 4. Multiple CEAC bimekizumab treatment-pathway vs. IL-17Ai treatment-pathways (axSpA population). BKZ and SEC cross at WTP £26,000 (probability of being cost-effective 33%). BKZ and IXE cross at WTP £31,000 (probability of being cost-effective 38%). Error bars indicate 95% confidence interval around the probability of being cost-effective. BKZ, bimekizumab treatment-pathway; CEAC, cost-effectiveness acceptability curve; IL, interleukin; IXE, ixekizumab treatment-pathway; nr-/r-axSpA, non-radiographic/radiographic axial spondyloarthritis; SEC, secukinumab treatment-pathway; WTP, willingness-to-pay.
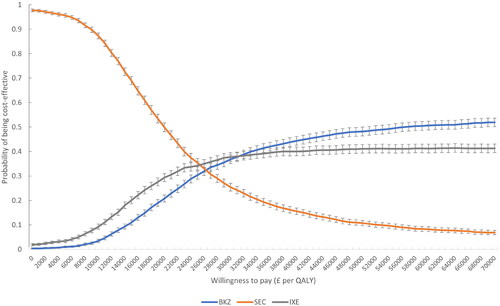
Table 4. Probabilistic base case, fully incremental cost-effectiveness results in axSpA population.
Table 5. Probabilistic base case, pairwise cost-effectiveness results in combined axSpA population.
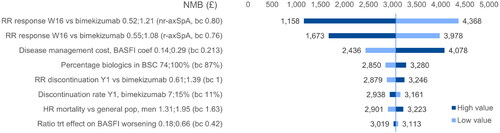
Figure 6. DSA BKZ vs. IXE (axSpA). NMB ranges obtained by applying the variations around the deterministic base case NMB to the probabilistic base case NMB. axSpA, axial spondyloarthritis; BASFI, Bath Ankylosing Spondylitis Functional Index; BKZ, bimekizumab treatment-pathway; NMB, net monetary benefit; nr, non-radiographic; trt, treatment; RR, relative risk.
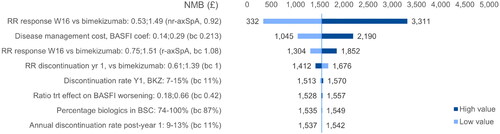
Table 6. Probabilistic scenarios analyses, axSpA population.