Figures & data
Table 1. ATC codes of available systemic menopausal hormone therapy (MHT) in Sweden 2000–2015.
Figure 1. Definition of drug use and duration of menopausal hormone therapy (MHT).
Note: X, MHT dispensation. Current MHT use was defined as at least one registered dispensation from the pharmacy within 4 months prior to the index date (diagnosis of pulmonary embolism [PE]), independently of previous dispensations or not (individuals 3–8). Women with a dispensation within 0–4 months, but not 5–12 months, before the index date were considered new users (individual 4). If they did not have any dispensation even further back in time, they were considered first ever users (individual 3). Ongoing treatment was defined as at least two dispensations per year consecutively. Women without any dispensation during the study period were considered non-users (individual 2). Women with dispensations that did not meet the criteria for continuous treatment were considered previous users and excluded from the analysis (individual 1).
![Figure 1. Definition of drug use and duration of menopausal hormone therapy (MHT).Note: X, MHT dispensation. Current MHT use was defined as at least one registered dispensation from the pharmacy within 4 months prior to the index date (diagnosis of pulmonary embolism [PE]), independently of previous dispensations or not (individuals 3–8). Women with a dispensation within 0–4 months, but not 5–12 months, before the index date were considered new users (individual 4). If they did not have any dispensation even further back in time, they were considered first ever users (individual 3). Ongoing treatment was defined as at least two dispensations per year consecutively. Women without any dispensation during the study period were considered non-users (individual 2). Women with dispensations that did not meet the criteria for continuous treatment were considered previous users and excluded from the analysis (individual 1).](/cms/asset/a719a1aa-c997-457b-99c4-9599e7c66b1b/icmt_a_2127352_f0001_c.jpg)
Figure 2. Flowchart showing the number of included and excluded cases of pulmonary embolism (PE) and controls. VTE, venous thromboembolism.
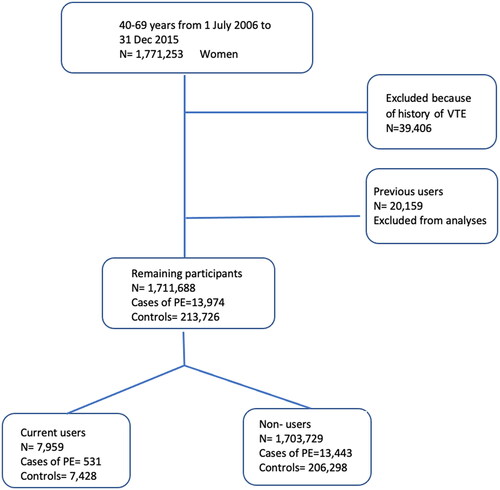
Table 2. Risk of pulmonary embolism (PE) in current, new and first ever users of menopausal hormone therapy (MHT), subclassified by route of administration and type of progestin used in combination with estrogen.
Table 3. Risk of pulmonary embolism (PE) depending on the duration of menopausal hormone therapy (MHT), subclassified by route of administration.
