Figures & data
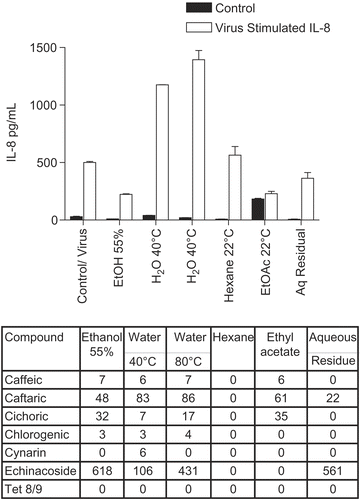
Figure 1. Anti-cytokine effects of E. purpurea root extracts. BEAS-2B cells were infected with rhinovirus type 14 (1 pfu/cell) for 60 min at 35°C, followed by 250 μg/mL of the indicated fraction of E. purpurea root extract. Controls received no virus. Solvent controls showed no effect. After 48 h, cell supernatants were removed for assay of IL-6 and IL-8 by standard ELISA tests. Standard curves were constructed for each experiment and the absorbance readings at 450 nm were converted into pg/mL. Composition of marker compounds is shown below the figure.
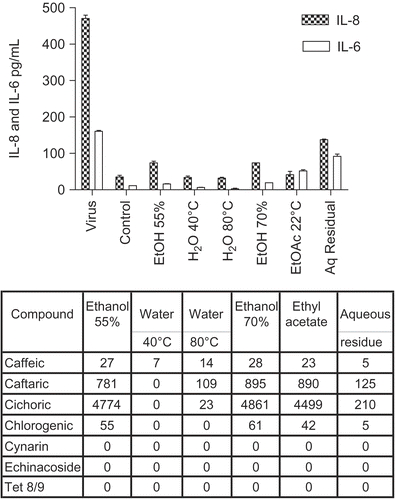
Figure 2. Anti-cytokine effects of E. purpurea leaf plus stem extracts. BEAS-2B cells were infected with rhinovirus type 14 (1 pfu/cell) for 60 min at 35°C, followed by 250 μg/mL of the indicated fraction of E. purpurea extract. Controls received no virus. After 48 h, cell supernatants were removed for assay of IL-6 and IL-8 by standard ELISA tests. Standard curves were constructed for each experiment and the absorbance readings at 450 nm were converted into pg/mL. Not all solvent fractions were available for these tests. Only IL-8 is shown in this graph. Similar results were obtained for IL-6. Composition of marker compounds is shown below the figure.
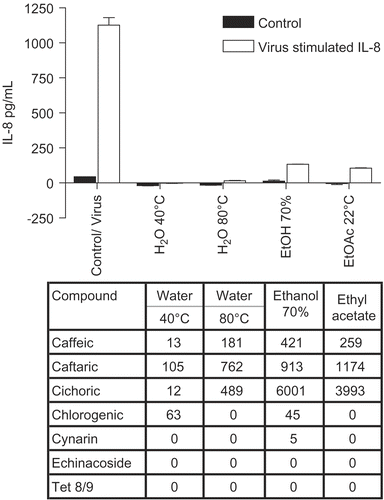
Figure 3. Anti-cytokine effects of E. purpurea flower extracts. BEAS-2B cells were infected with rhinovirus type 14 (1 pfu/cell) for 60 min at 35°C, followed by 250 μg/mL of the indicated fraction of E. purpurea flower extract. Controls received no virus. After 48 h, cell supernatants were removed for assay of IL-6 and IL-8 by standard ELISA tests. Standard curves were constructed for each experiment and the absorbance readings at 450 nm were converted into pg/mL. Only IL-8 is shown in this graph; similar results were obtained for IL-6. Composition of marker compounds is shown below the figure.
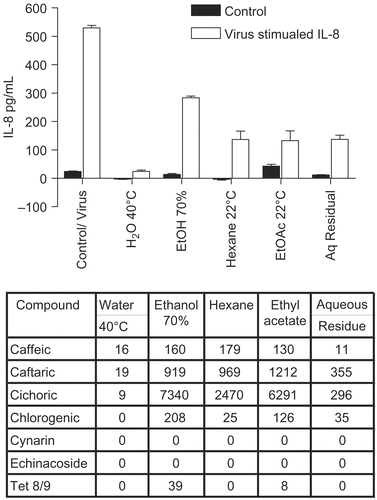
Figure 4. Anti-cytokine effects of polysaccharide-enriched fractions. BEAS-2B cells were infected with rhinovirus type 14 (1 pfu/cell) for 60 min at 35°C, followed by 250 μg/mL of the indicated fraction derived from E. purpurea aqueous pressed juice. Controls received no virus. After 48 h, cell supernatants were removed for assay of IL-6 and IL-8 by standard ELISA tests. Standard curves were constructed for each experiment and the absorbance readings at 450 nm were converted into pg/mL. Polysaccharide-enriched fraction, P50 fraction (polysaccharides > 100 kDa) isolated from pressed juice; AGP fraction, arabinogalactan isolated by SEC 1 column from the polysaccharide fraction.
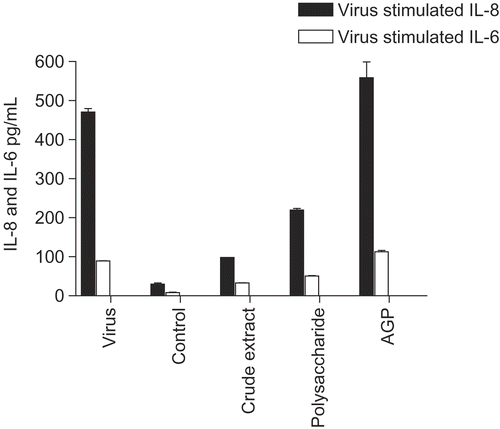
Figure 5. Anti-cytokine effects of E. angustifolia root extracts. BEAS-2B cells were infected with rhinovirus type 14 (1 pfu/cell) for 60 min at 35°C, followed by 250 μg/mL of the indicated fraction of E. angustifolia root extract. Controls received no virus. After 48 h, cell supernatants were removed for assay of IL-6 and IL-8 by standard ELISA tests. Standard curves were constructed for each experiment and the absorbance readings at 450 nm were converted into pg/mL. Only IL-8 is shown; the results for IL-6 were similar. Not all solvent fractions were available for this experiment. Composition of marker compounds is shown below the figure.
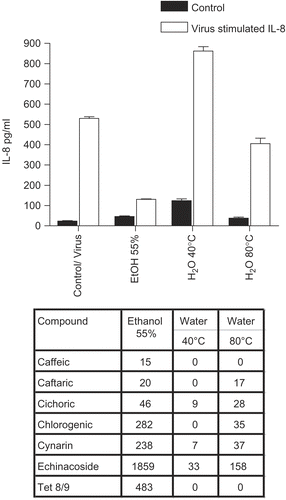
Figure 6. Anti-cytokine effects of E. pallida root extracts. BEAS-2B cells were infected with rhinovirus type 14 (1 pfu/cell) for 60 min at 35°C, followed by 250 μg/mL of the indicated fraction of E. pallida root extract. Controls received no virus. After 48 h, cell supernatants were removed for assay of IL-6 and IL-8 by standard ELISA tests. Standard curves were constructed for each experiment and the absorbance readings at 450 nm were converted into pg/mL. Only IL-8 data are shown; the results for IL-6 were similar. Composition of marker compounds is shown below the figure.