Figures & data
Table 1. Isoforms tested, marker reactions, incubation conditions, and Km used in the inhibition study.
Figure 1. Effects of Friedelin (100 μM) on the activity of CYP450 enzymes in pooled HLMs. All data represent mean ± SD of the triplicate incubations. *p < 0.05, significantly different from the negative control. Negative control: incubation systems without Friedelin; Friedelin: incubation systems with Friedelin; positive control: incubation systems with their corresponding positive inhibitors.
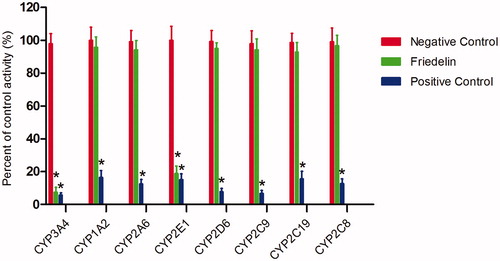
Figure 2. Lineweaver–Burk plots (A) and the secondary plot for Ki (B) of effects of Friedelin on CYP3A4 catalyzed reactions (testosterone 6β-hydroxylation) in pooled HLM. Data are obtained from a 30 min incubation with testosterone (20–100 μM) in the absence or presence of Friedelin (0–30 μM). All data represent mean ± SD of the triplicate incubations.
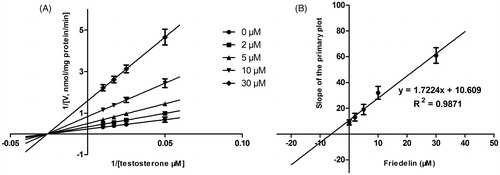
Figure 3. Lineweaver–Burk plots (A) and the secondary plot for Ki (B) of effects of Friedelin on CYP2E1 catalyzed reactions (chlorzoxazone 6-hydroxylation) in pooled HLM. Data are obtained from a 30 min incubation with chlorzoxazone (25–200 μM) in the absence or presence of Friedelin (0–50 μM). All data represent mean ± SD of the triplicate incubations.
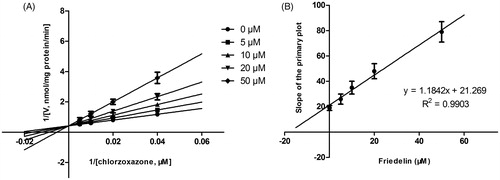
Figure 4. Time-dependent inhibition investigations of CYP3A4 catalyzed testosterone 6β-hydroxylation and CYP2E1 catalyzed chlorzoxazone 6-hydroxylation reactions by Friedelin (20 μM). All data represent mean ± SD of the triplicate incubations.
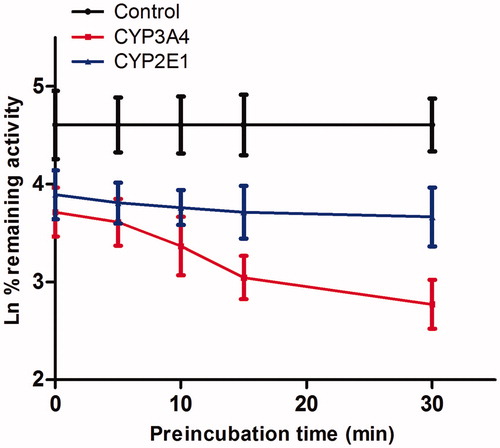
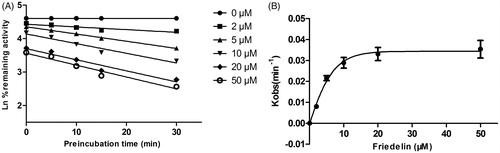
Figure 5. Time and concentration-inactivation of microsomal CYP3A4 catalyzed testosterone 6β-hydroxylation by Friedelin in the presence of NADPH. The initial rate constant of inactivation of CYP3A4 by each concentration (Kobs) was determined through linear regression analysis of the natural logarithm of the percentage of remaining activity versus preincubation time (A). The Ki and Kinact values were determined through nonlinear analysis of the Kobs versus the Friedelin concentration (B).