Figures & data
Table 1. Baseline characteristics of REWIND participants.
Figure 1. Percentage of patients meeting relevant clinical targets at baseline and at two years in (a) patients with history of cardiovascular disease at baseline, and (b) patients without history of cardiovascular disease at baseline. This subgroup analysis includes a subset of intention-to-treat (ITT) population excluding patients with missing data at baseline or two years for HbA1c, systolic blood pressure, LDL-cholesterol, and concomitant medications or baseline history of CVD either missing or unknown. Abbreviations: ACE: angiotensin-converting enzyme; ARBs: angiotensin-receptor blockers; CVD: cardiovascular disease; HbA1c: glycated hemoglobin A1c; LDL: low-density lipoprotein; N: total number of participants in each gender group; SBP: systolic blood pressure.
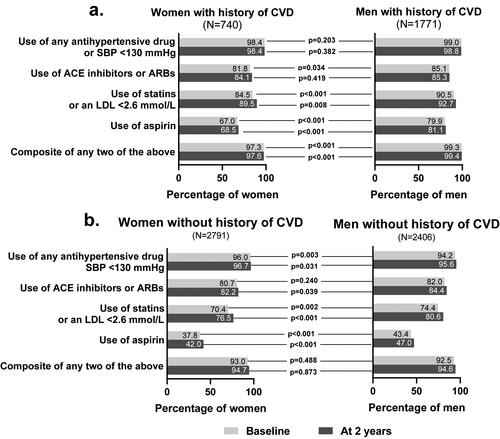
Figure 2. Gender differences in major cardiovascular event (MACE) outcome. (a) Model adjusted for gender, randomized treatment, and selected baseline characteristics. (b) Model adjusted for gender and randomized treatment. This analysis was performed on the full ITT population. Baseline characteristics selected for adjustment for each outcome in are listed in Table S4. Abbreviations: CI: confidence interval; CV: cardiovascular; HF: heart failure; HR: hazard ratio; ITT: intention-to-treat; MACE: major adverse cardiovascular events; MI: myocardial infarction n: number of patients in each category; N: total number of participants in each gender group; p-yr: patient-year.
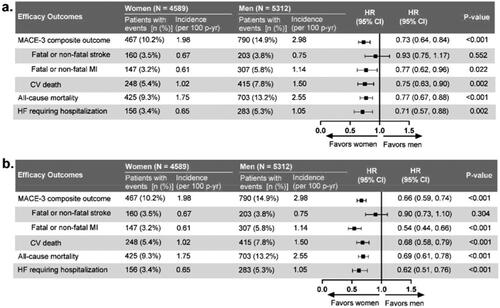
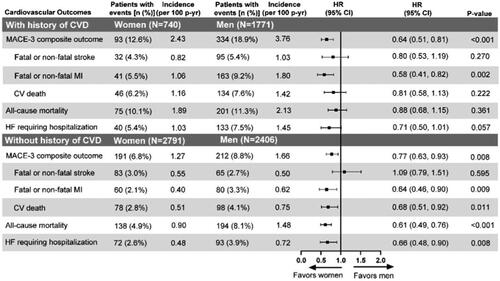
Figure 3. Time-to-event analysis of the cardiovascular risk outcomes with gender comparison. This subgroup analysis includes a subset of ITT population excluding patients with missing data at baseline or two years for HbA1c, SBP, LDL-cholesterol, and concomitant medications or baseline history of CVD either missing or unknown. Abbreviations: CI: confidence interval; CV: cardiovascular; CVD: cardiovascular disease; HbA1c: glycated hemoglobin A1c; HF: heart failure; HR: hazard ratio; ITT: intention-to-treat; LDL: low-density lipoproteins; n: number of patients in each category; N: total number of participants in each gender group; MACE: major cardiovascular events; MI: myocardial infarction; p-yr: patient year; SBP: systolic blood pressure.
Supplemental Material
Download MS Word (41.8 KB)Data availability statement
The data that support the findings will be disclosed only upon request and approval of the proposed use of the data by a review committee. All the data for the present report came from the Population Health Institute (PHRI) in Hamilton, Canada, who also did all the data analysis for the REWIND trial. The REWIND data sharing policy is described in the Supplemental Material.
