Figures & data
Figure 1. Patient flow. DAS28-4/ESR, 28-joint count Disease Activity Score based on four erythrocyte sedimentation rate; MHAQ, modified Health Assessment Questionnaire.
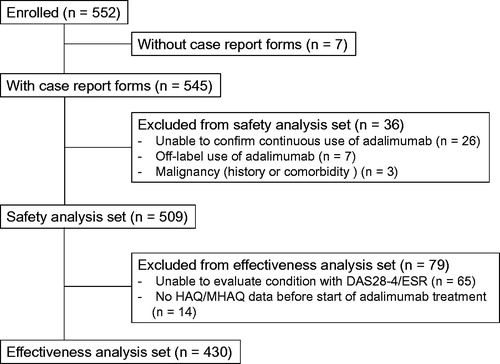
Table 1. Baseline patient characteristics (n = 509).
Figure 2. Treatment continuation rate over time. The Kaplan–Meier method was used to estimate the treatment continuation rate throughout the observation period.
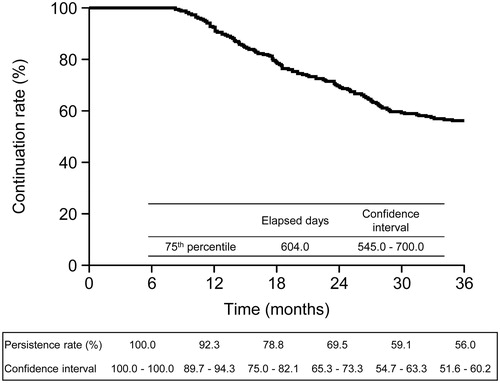
Figure 3. Incidence rates of ADRs, SADRs, infection and serious infection over time. (a) ADR and SADR. (b) Infection and serious infection. The incidence rate was calculated by dividing the number of patients who developed ADRs, SADRs, infection and serious infection during each period by the number of patients who were followed at the beginning of each period. ADR: adverse drug reaction; SADR: serious adverse drug reaction.
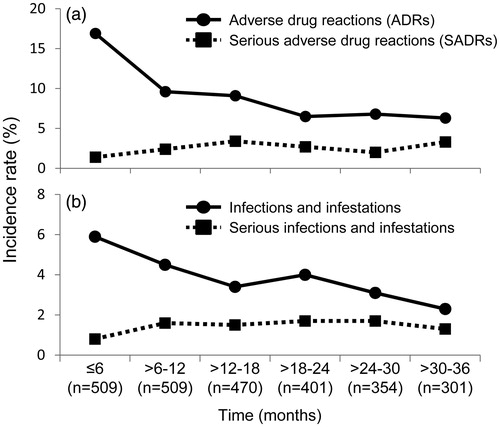
Table 2. Incidences of adverse drug reactions and serious adverse drug reactions in the safety analysis set (n = 509).
Table 3. Malignancies reported in this study.
Table 4. Factors associated with development of ADRs or SADRs.
Figure 4. Changes in patient distribution in disease activity status over time. Patients were categorized into high, moderate, low disease activity or remission groups using DAS28-4/ESR scores and the last observation carried forward method. DAS28-4/ESR, 28-joint count Disease Activity Score based on four erythrocyte sedimentation rate. *p < .001 for significant linear trend by Cochran–Armitage test.
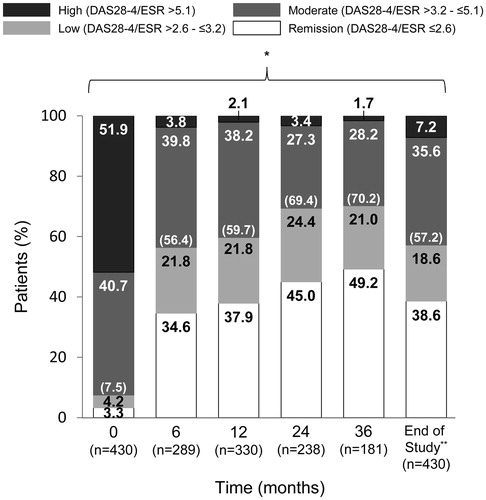
Figure 5. Changes in patient distribution in MHAQ score over time. Patients were categorized using MHAQ score and the last observation carried forward method. MHAQ: modified Health Assessment Questionnaire. *p < .001 for significant linear trend by Cochran–Armitage test.
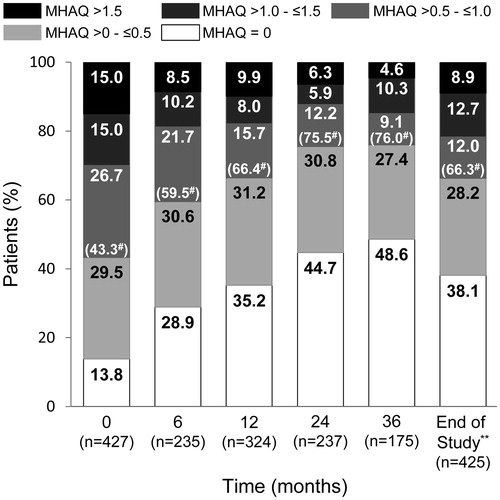
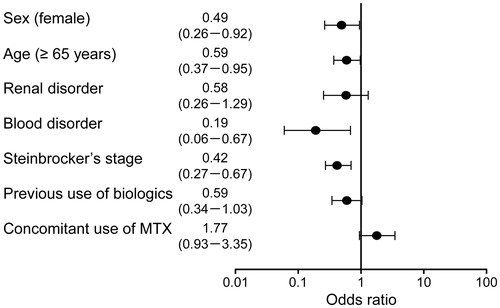
Figure 6. Factors associated with DAS28-4/ESR remission at the end of the study using logistic regression analysis. The Hosmer–Lemeshow goodness-of-fit test, p = .4548. DAS28-4/ESR: 28-joint count Disease Activity Score based on four erythrocyte sedimentation rate; MTX: methotrexate.