Figures & data
Figure 1. Sample selection flow chart. DMARD: disease-modifying antirheumatic drug; PsA: psoriatic arthritis.
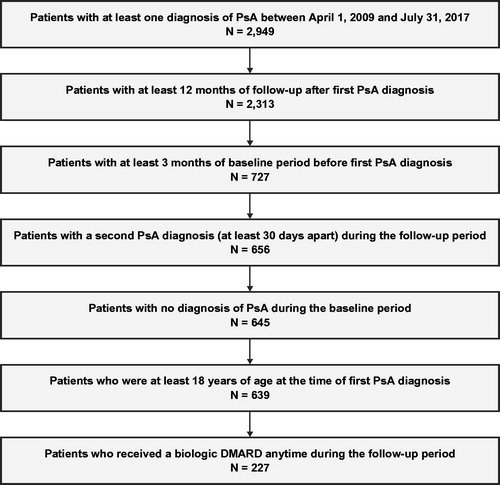
Table 1. Patient demographics and clinical characteristics at baseline.
Table 2. Comorbidity burden at baseline using the charlson comorbidity index and other comorbidities of interest.
Figure 2. Treatment sequencing following the top five first-line combinations for all patients with PsA diagnosis. cs: conventional synthetic; DMARD: disease-modifying antirheumatic drug; NSAID: nonsteroidal anti-inflammatory drug; PsA: psoriatic arthritis. Notes. Sixty-nine patients (10.8%) had no observed treatment during available follow-up, and 24.9% had other treatment sequences not shown in the top 5 treatments. ‘Other treatments’ comprises second-line treatments received by fewer than five patients. Patients in the NSAID second-line treatment category following first-line treatment of NSAID only are patients who discontinued and then restarted NSAID treatment.
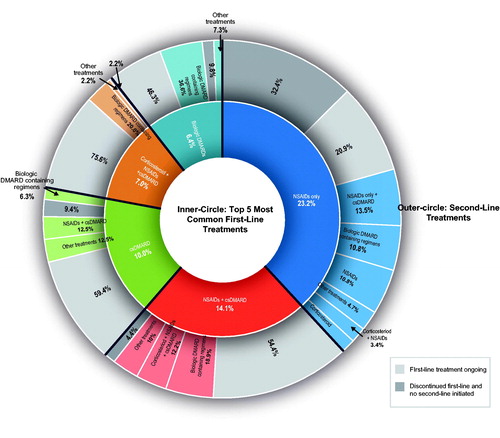
Figure 3. Logistic regression analysis to assess factors associated with receipt of biologic DMARDs in the 12 months after diagnosis of PsA. CCI: Charlson Comorbidity Index; CI: confidence interval; cs: conventional synthetic; DMARD: disease-modifying antirheumatic drug; NSAID: nonsteroidal anti-inflammatory drug; PsA: psoriatic arthritis; ref.: reference.
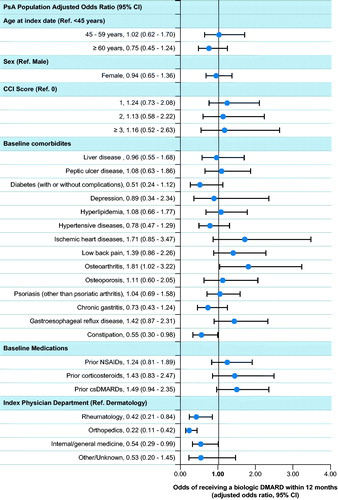
Table 3. Overall treatment categories during the 12-month follow-up perioda.
Table 4. First treatments administered to patients during the 12-month follow-up perioda.
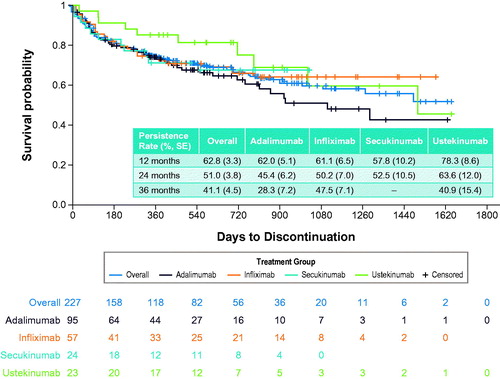
Table 5. Treatment patterns during all available follow-up among patients receiving biologic DMARDsa.
Table 6. All-Cause and Other specific health care resource utilization and costs during anytime in the follow-up period.