Figures & data
Figure 1. Patient disposition.
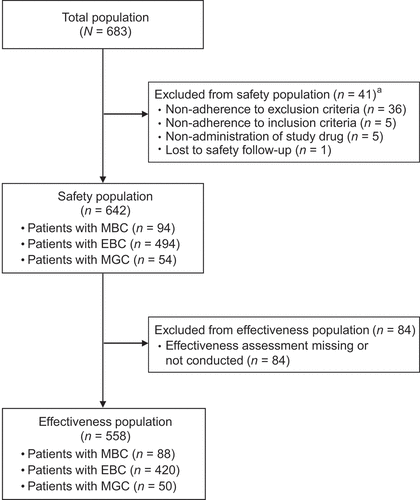
Table 1. Summary of patient demographics and baseline characteristics by indication (safety population).
Table 2. Summary of the four most common ADRs and serious ADRs by preferred term, per indication (safety population).
Table 3. Summary of AEs and ADRs of special interest, per indication (safety population).
Table 4. Summary of effectiveness, by indication (effectiveness population).
CT-P6 4.1 study_supplementary materials.docx
Download MS Word (40.9 KB)Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.

