Figures & data
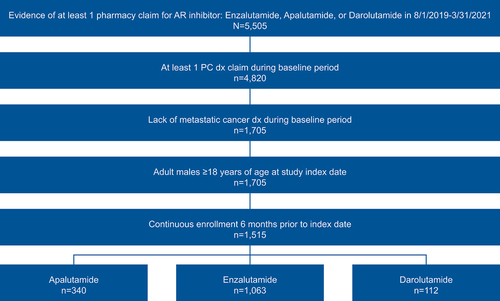
Figure 1. Patient identification methodology.
Table 1. Baseline demographics.
Table 2. Charlson Comorbidity Index and select comorbidities.
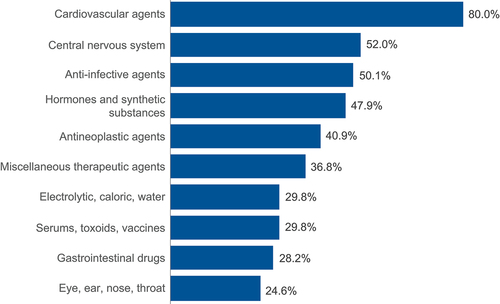
Figure 5. pDDIs out of the 100 most commonly prescribed concomitant medications by AR inhibitor.
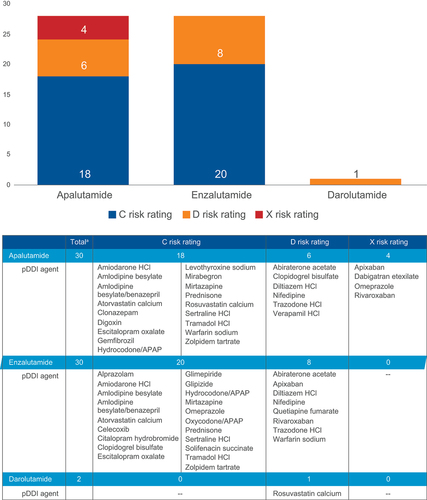
Table 3. Implicated enzymes for pDDIs.