Figures & data
Figure 1. The three stages of the market maturity framework.a
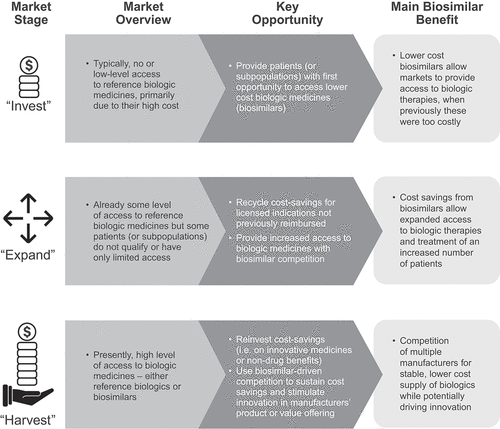
Figure 2. Estimated value of biologic products faced with loss of exclusivity in Europe (2010‒2029). (Reproduced from, the impact of biosimilar competition in Europe, Troein, P., Newton, M., Scott, K. © IQVIA Inc. 2020; by permission of IQVIA Inc.) [Citation8].
![Figure 2. Estimated value of biologic products faced with loss of exclusivity in Europe (2010‒2029). (Reproduced from, the impact of biosimilar competition in Europe, Troein, P., Newton, M., Scott, K. © IQVIA Inc. 2020; by permission of IQVIA Inc.) [Citation8].](/cms/asset/f92edf7e-3bb7-4dec-a86c-48443e6cbd9a/ierp_a_2310684_f0002_b.gif)
Interactive summary
Download PDF (1.5 MB)Data availability statement
Not applicable.
