Figures & data
Table 1. Four main algorithms used to assess the potential association between bendamustine and AEs.
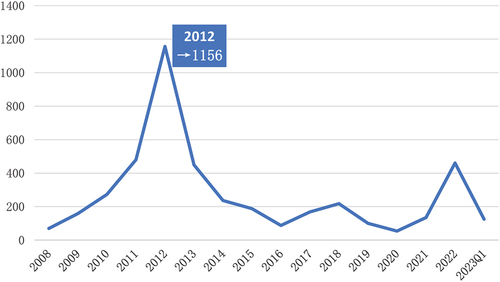
Table 2. Clinical characteristics of bendamustine use reported in the FAERS Database (January 2008 to March 2023).
Table 3. Top 10 types of adverse reactions to bendamustine.
Table 4. ADR events involving system organs.
Table 5. Comparison of Signal Intensity Reported for Bendamustine at preferred term levels by SOC classification.