Figures & data
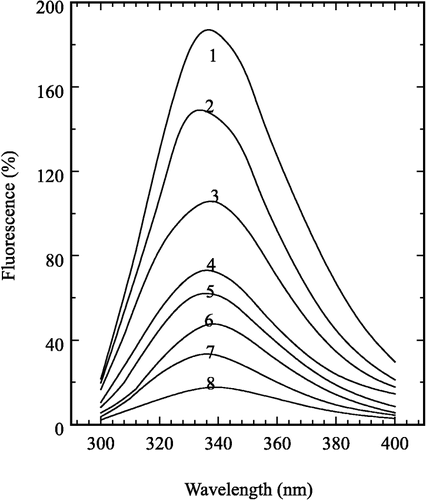
Figure 1 Determination of the kinetic parameters of prawn NAGase. Conditions were 2 ml system containing 0.15 M NaAc-HAc buffer (pH 5.2) and different concentrations of pNP-β-D-GlcNAc at 37°C. The enzyme final concentration was 0.020 μM. The inset is a Lineweaver-Burk plot for the determination of Km and Vm for NAGase on the hydrolysis of pNP-β-D-GlcNAc.
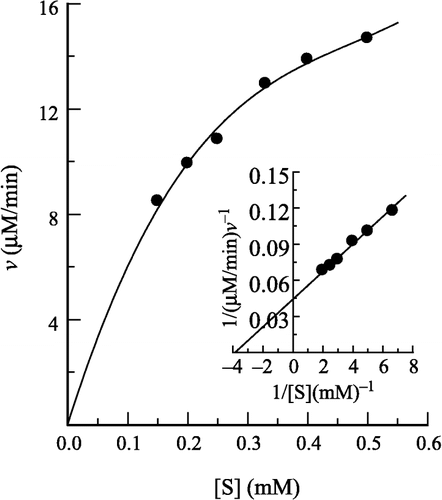
Figure 2 Inhibition of the enzyme in different concentration of H2O2 solution. Conditions were as same as in Figure 1 except that pNP-β-D-GlcNAc concentration was 0.5 mM with different concentrations of H2O2. The enzyme final concentration was 0.020 μM.
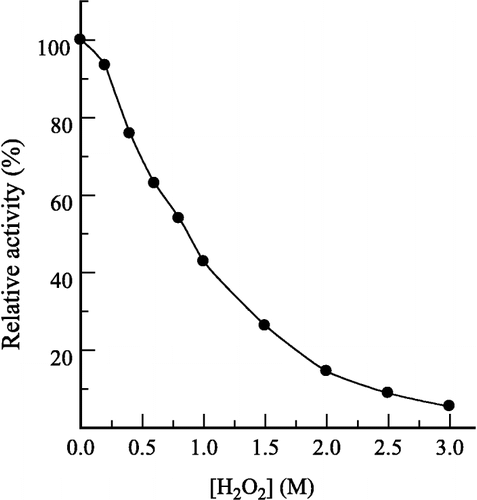
Figure 3 Effects of prawn NAGase concentration on its activity at different concentrations of H2O2. The concentrations of H2O2 for curves 0–5 were 0, 0.2, 0.4, 0.6, 0.8 and 1.0 M, respectively. Assay conditions were same as in Figure 2.
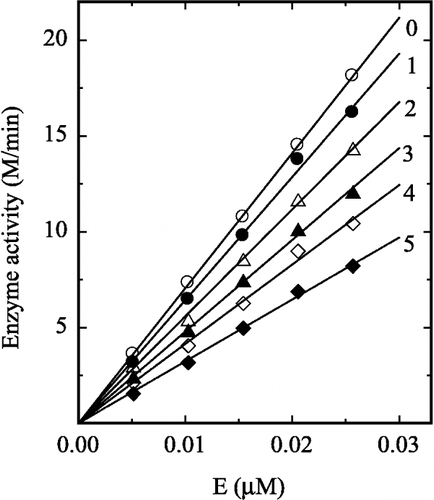
Figure 4 Lineweaver-Burk plots for the enzyme in different concentrations of H2O2. The H2O2 concentration for lines 0–4 was 0, 0.2, 0.4, 0.6 and 0.8 M, respectively. Conditions were the same as in Figure 1.
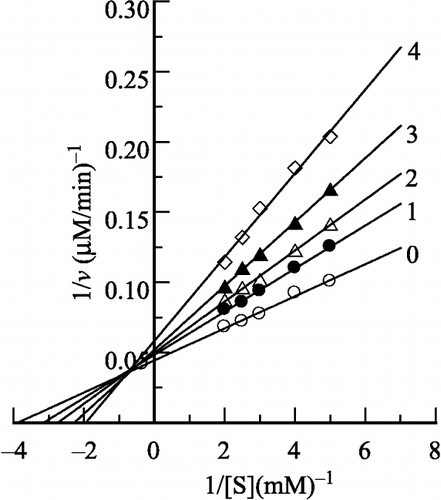
Figure 5 Course of inhibition of the enzyme in different concentrations of H2O2. The assay conditions were the same as in Figure 1 except that the concentration of pNP-β-D-GlcNAc was 0.4 mM. (a) Substrate reaction course. The final H2O2 concentrations for curves 0–4 were 0, 0.2, 0.4, 0.6, and 0.8 M, respectively. (b) Semilogarithmic plots of ln([P]calc − [P]t) against time. Data were taken from curves 1–4 in (a).
![Figure 5 Course of inhibition of the enzyme in different concentrations of H2O2. The assay conditions were the same as in Figure 1 except that the concentration of pNP-β-D-GlcNAc was 0.4 mM. (a) Substrate reaction course. The final H2O2 concentrations for curves 0–4 were 0, 0.2, 0.4, 0.6, and 0.8 M, respectively. (b) Semilogarithmic plots of ln([P]calc − [P]t) against time. Data were taken from curves 1–4 in (a).](/cms/asset/a4771fe4-d418-476a-9c44-d28172c6b35e/ienz_a_114862_f0006_b.gif)
Figure 6 Course of inhibition at different substrate concentration in the presence of 0.40 M H2O2. (a) Curves 1–5 are progress curves with 0.50, 0.40, 0.33, 0.25 and 0.20 mM pNP-β-D-GlcNAc, respectively. (b) Semilogrithmic plot of ln ([P]calc − [P]t) against time. Data were taken from curves 1–5 in (a).
![Figure 6 Course of inhibition at different substrate concentration in the presence of 0.40 M H2O2. (a) Curves 1–5 are progress curves with 0.50, 0.40, 0.33, 0.25 and 0.20 mM pNP-β-D-GlcNAc, respectively. (b) Semilogrithmic plot of ln ([P]calc − [P]t) against time. Data were taken from curves 1–5 in (a).](/cms/asset/b55147f4-4bb4-4538-8949-75b2d1befe1c/ienz_a_114862_f0007_b.gif)
Figure 7 Secondary plots of the slopes of the semilogarithmic plots versus [H2O2] for a series of fixed substrate concentrations. The data for curve 2 is from Figure 5b (at 0.40 mM substrate). Substrate concentrations for curves 1–5 were 0.50, 0.40, 0.33, 0.25, 0.20 mM, respectively.
![Figure 7 Secondary plots of the slopes of the semilogarithmic plots versus [H2O2] for a series of fixed substrate concentrations. The data for curve 2 is from Figure 5b (at 0.40 mM substrate). Substrate concentrations for curves 1–5 were 0.50, 0.40, 0.33, 0.25, 0.20 mM, respectively.](/cms/asset/aa460b45-0a24-4294-9c30-dc1540e1b0ab/ienz_a_114862_f0008_b.gif)
Table I. Microscopic rate constants of the inactivation of prawn NAGase in H2O2 solution.
![Figure 8 Plot of A/v versus 1/[S]. The values of v and A were obtained from Figures 1 and 7.](/cms/asset/04bced5b-f6cd-4b70-9a98-a1919a26c6c5/ienz_a_114862_f0009_b.gif)