Figures & data
Figure 1 Inhibitory effects of PLE on FAS activity. The inhibition of the OA (•) and the KR (♦) of FAS in the presence of various concentrations of PLE were measured. The IC50 values were means of three experiments obtained from the figures.
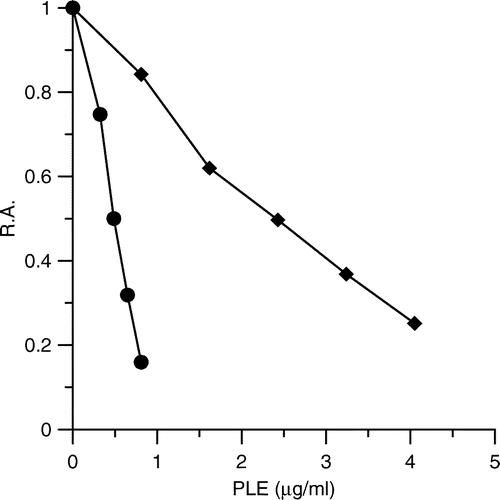
Figure 2 Double-reciprocal plots for inhibition of FAS by PLE. (A) The OA of FAS was measured. Acetyl-CoA was the variable substrate. The concentrations of malonyl-CoA and NADPH were fixed at 0.37 mM and 1.34 mM. The concentrations of PLE were: 0 μg/ml (•); 0.33 μg/ml (♦); 0.49 μg/ml (▴); 0.81 μg/ml (+) respectively. (B) The KR of FAS was measured. NADPH was the variable substrate. The concentrations of Acetyl-CoA and malonyl-CoA were fixed at 0.192 mM and 0.37 mM. The concentrations of PLE were: 0 μg/ml (•); 0.98 μg/ml (♦); 1.95 μg/ml (▴); 2.93 μg/ml (+) respectively.
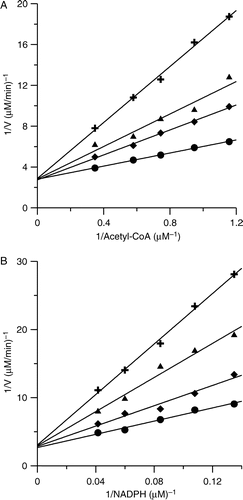
Table I. Inhibition pattern and inhibition parameters for the inhibition of FAS by PLE and avicularin with different substrates.
Figure 3 Semi-logarithmic plot of time-dependent irreversible inhibition of FAS in the presence of PLE. PLE (18 μg /ml) was mixed with FAS solution (0.15 μM) and aliquots of the mixture were loaded on a prepared 1.2 ml Sephedex G25 (coarse) column and centrifuged at predetermined time intervals when the enzyme fraction was collected to measure the R.A. of FAS. The plot represents Ln (R.A.) versus time. The fast-phase (▴) inhibition occurred from 0 to 5 min (the contribution of slow phase was subtracted), and the slow-phase (•) ranged from 5 to 80 min. The linear slopes of the two lines gave the apparent first-order rate constants.
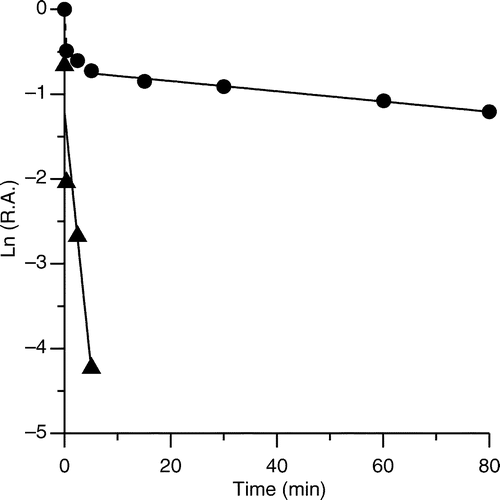
Figure 4 Degree of FAS inhibition by different concentration of PLE at 0.37 min and 2.5 min. (A) FAS solutions (0.15 μM) were mixed with PLE at 18 μg/ml and 27 μg/ml respectively. Aliquots of the mixture with or without gel filtration on Sephedex G25 were taken to assay the R.A. of FAS. FAS mixed with the solvent was used as control (white bar). The R.A. at 0.37 min (black bar) and 2.5 min (forward slash bar) are shown respectively. FG = Gel Filtration; NOGF = No Gel Filtration. (B) PLE (18 μg/ml) was mixed with FAS solutions of 0.15 μM and 0.82 μM. The R.A. without gel filtration at 0.37 min (black bar) and 2.5 min (forward slash bar) are shown. FAS mixed with the solvent was used as control (white bar). Each histogram was the mean of three experiments.
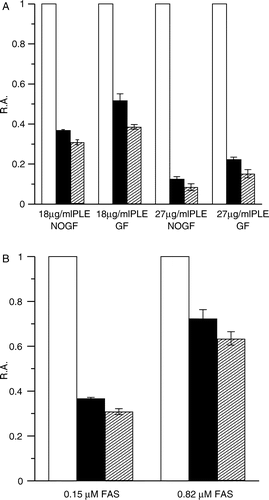
Table II. The IC50 values of some glycosylated flavonoids on the OA and KR of FAS.