Figures & data
Figure 1 Role of type 3 17β-HSD in the transformation of less potent androgen Δ4-dione into active androgens T and DHT, using the cofactor nicotinamide adenine dinucleotide phosphate (NADPH).
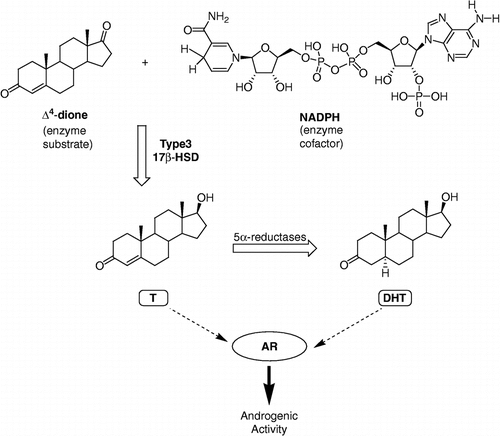
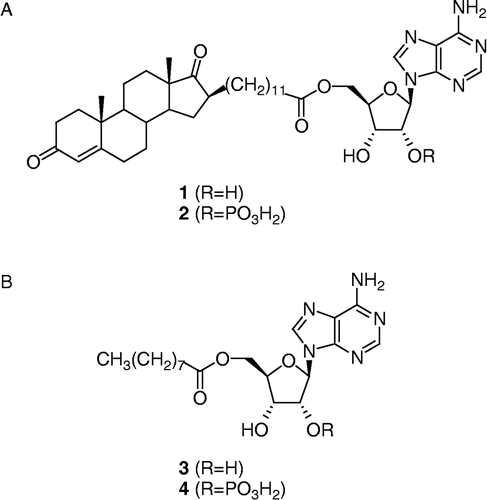
Figure 2 (A) Bisubstrate inhibitor and phosphorylated bisubstrate inhibitor of type 3 17β-HSD, compounds 1 and 2. (B) Cofactor-like compounds 3 and 4.
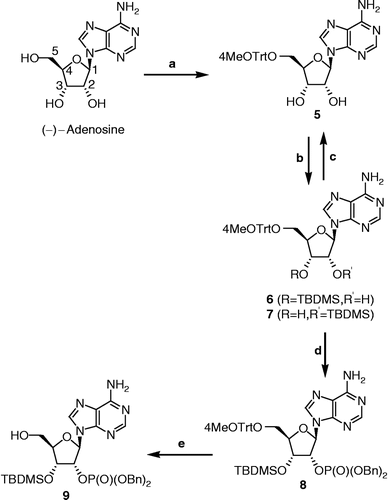
Scheme 1 Synthesis of phosphorylated adenosine 9. Reagents, conditions and yields: (a) 4-MeOTrtCl, DMF, rt, 3 days (60%); (b) TBDMSCl, imidazole, DMF, rt, 20 h (6: 40%, 7: 37%); (c) 7, TBAF, THF, 0°C, 2 h (99%); (d) i. 6, dibenzyl diisopropylphosphoramidite, 1H-tetrazole, CH2Cl2, 0°C, 30 min; ii. m–CPBA, 0°C, 30 min (55%); (e) dichloroacetic acid, CH2Cl2, rt, 4 h (91%).
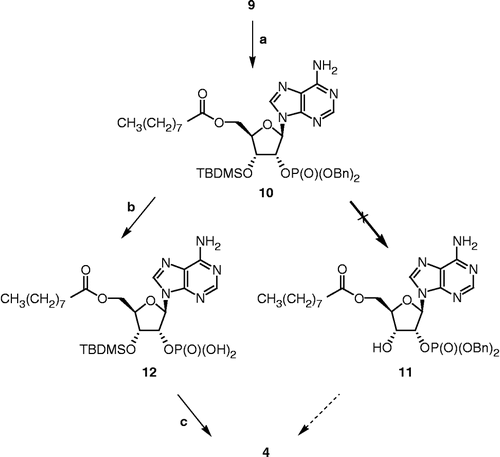
Scheme 2 Synthesis of 4. Reagents, conditions and yields: (a) i. nonanoic acid, EDCI, DMAP, DMF, rt, 30 min; ii. 9, DMF, rt, 16 h (70%); (b) Pd(OH)2, cyclohexene, MeOH, reflux, 30 min; (c) HClg, CH2Cl2, rt, 30 min (28% for two steps).
Scheme 3 Synthesis of 2. Reagents, conditions and yields: (a) i. 13, EDCI, DMAP, DMF, rt, 30 min; ii. 9, DMF, rt, 16 h (47%); (b) p-TSA, CH2Cl2, MeOH, rt, 1 h (59%); (c) Pd(OH)2, cyclohexene, MeOH, reflux, 30 min; (d) HClg, CH2Cl2, rt, 30 min (56% for two steps).
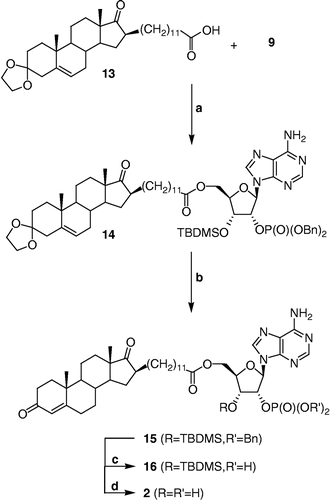
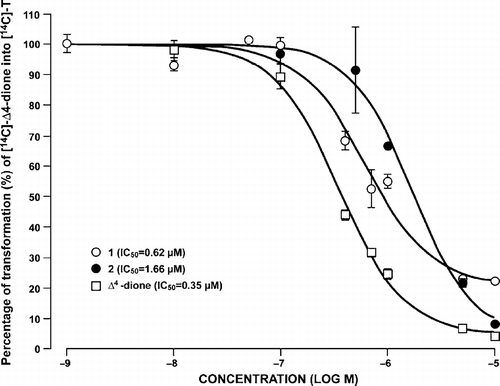
Figure 3 Inhibition of the transformation of [14C]-Δ4-dione into [14C]-T by compounds 1–4 and unlabeled Δ4-dione at different concentrations in homogenated HEK-293 cells overexpressing type 3 17β-HSD. A: First enzymatic assay. B: Second enzymatic assay. See Experimental Section for more details on the experimental conditions used.
![Figure 3 Inhibition of the transformation of [14C]-Δ4-dione into [14C]-T by compounds 1–4 and unlabeled Δ4-dione at different concentrations in homogenated HEK-293 cells overexpressing type 3 17β-HSD. A: First enzymatic assay. B: Second enzymatic assay. See Experimental Section for more details on the experimental conditions used.](/cms/asset/68057ffc-a621-455a-95ba-4da77463853f/ienz_a_205046_f0006_b.gif)