Figures & data
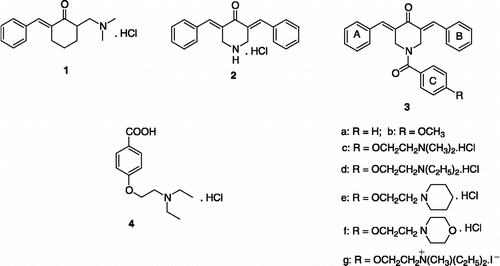
Table I. Stimulation of fyn kinase by 2, 3b-g and 4.
Table II. Effects of low concentrations of 2, 3a,b and 4 on fyn kinase.
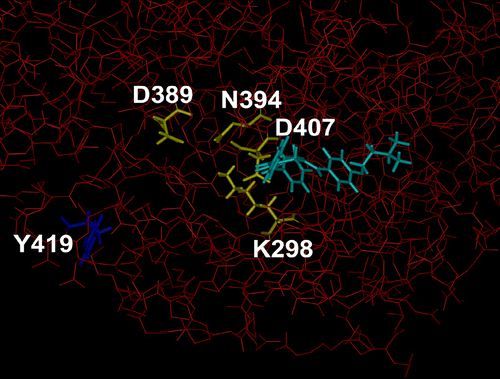
Figure 2 Structure of part of fyn kinase in complex with 3c. The heavy atoms of fyn 60-536 are coloured red and displayed as thin sticks. The residues in fyn that participate in coordinating the magnesium ion and the α- and β-phosphates of ATP (fyn K298, N394, and D407) and the proton acceptor site (fyn D389) are coloured yellow and displayed as thick sticks. The autocatalytic phosphorylation site in fyn, Y419, is coloured dark blue. The docked compound 3c is coloured light-blue. The docked compounds do not directly interact with the proton acceptor site (fyn D389) nor the residues coordinating the phosphate-magnesium ion (side chain atoms of fyn K298, N394, and D407), rather they interact with the residues coordinating the adenosine moiety of ATP, and include backbone atoms of K298 andD407. Thus, the presence of the compounds may alter the orientation of the side chains of K298 and D407 in fyn and thereby effect ATP binding. (Please see colour online)