Figures & data
Table I. ki and αi values for HRP and MP-11 were obtained for a time course of 10 min of the progress curves data at 27°C, pH=7.0, phosphate buffer 5.0 mM.
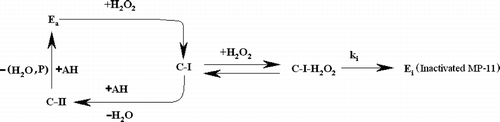
Figure 1 Progress curves for suicide inactivation of MP-11 by hydrogen peroxide, as the variation of guaiacol concentration versus reaction time, according to Equation (4). Inactivation rate constant, ki and the initial activity of MP-11, αo, were estimated by successive approximation and non-linear fitting of the experimental data into Equation (4). Solid lines indicate the calculated curve data using the obtained ki and αo parameters in Equation (4).Values of the kinetic parameters are shown in . Each parameter was systematically adjusted to produce best fit curves that gave a minimum value of the sum-of-squares of residuals (SSR, as the differences between observed and calculated values of progress curves, i.e. SSR = Σ(yobs.–ycalc.)2). An Excel Solver program was used for this purpose. A detailed description of the suicide-peroxide inactivation model is given in references [Citation33] and [Citation37]. Reactions were started by adding hydrogen peroxide to the mixture of AH and MP-11 (1.0 μM) having an initial activity of 0.3 min− 1, time course of about 30 min at 27°C, phosphate buffer 5.0 mM, pH = 7.0. a) [AH] = 200 μM, [H2O2] = 1.0 mM, ki = 0.487 min− 1 b) [AH] = 100 μM, [H2O2] = 1.0 mM, ki = 0.491 min− 1, c) [AH] = 100 μM, [H2O2] = 0.40 mM, ki = 0.120 min− 1 d) The hypothetical curve with ki = 0 (using value of ki = 10− 8 for solving Equation (4)), i.e. without suicidal effects of peroxide. Here [AH]o and αo were considered to be the same as state (c). AH concentration was followed from the absorbance of the reaction mixture at 470 nm using Equations (2) and (3). In order to reach the stationary state of the reaction, a dead time of 30 seconds was considered for processing the progress curves data.
![Figure 1 Progress curves for suicide inactivation of MP-11 by hydrogen peroxide, as the variation of guaiacol concentration versus reaction time, according to Equation (4). Inactivation rate constant, ki and the initial activity of MP-11, αo, were estimated by successive approximation and non-linear fitting of the experimental data into Equation (4). Solid lines indicate the calculated curve data using the obtained ki and αo parameters in Equation (4).Values of the kinetic parameters are shown in Table I. Each parameter was systematically adjusted to produce best fit curves that gave a minimum value of the sum-of-squares of residuals (SSR, as the differences between observed and calculated values of progress curves, i.e. SSR = Σ(yobs.–ycalc.)2). An Excel Solver program was used for this purpose. A detailed description of the suicide-peroxide inactivation model is given in references [Citation33] and [Citation37]. Reactions were started by adding hydrogen peroxide to the mixture of AH and MP-11 (1.0 μM) having an initial activity of 0.3 min− 1, time course of about 30 min at 27°C, phosphate buffer 5.0 mM, pH = 7.0. a) [AH] = 200 μM, [H2O2] = 1.0 mM, ki = 0.487 min− 1 b) [AH] = 100 μM, [H2O2] = 1.0 mM, ki = 0.491 min− 1, c) [AH] = 100 μM, [H2O2] = 0.40 mM, ki = 0.120 min− 1 d) The hypothetical curve with ki = 0 (using value of ki = 10− 8 for solving Equation (4)), i.e. without suicidal effects of peroxide. Here [AH]o and αo were considered to be the same as state (c). AH concentration was followed from the absorbance of the reaction mixture at 470 nm using Equations (2) and (3). In order to reach the stationary state of the reaction, a dead time of 30 seconds was considered for processing the progress curves data.](/cms/asset/c4f0dcae-54dc-4f27-bb6b-2ebde7f964c4/ienz_a_226977_f0002_b.gif)
Figure 2 a) Variation of ki with initial concentration of guaiacol, AH. ki values were obtained (27°C, phosphate buffer 5.0 mM, pH = 7.0) from the related progress curves and Equation (4) as described in legend of Figure 1. as a function of activity (concentration) of MP-11, αo. Values of ki and αo were obtained (27°C, phosphate buffer 5.0 mM, pH = 7.0, [H2O2] = 1.0 mM) from the related progress curves and Equation (4) as described in the text and in the legend of Figure 1. c) αo as a function of concentration of hydrogen donor substrate, [AH]. Values of αo were obtained (27°C, phosphate buffer 5.0 mM, pH = 7.0, [H2O2] = 1.0 mM) from the related progress curves and Equation (4) as described in the text and in the legend of Figure 1.
![Figure 2 a) Variation of ki with initial concentration of guaiacol, AH. ki values were obtained (27°C, phosphate buffer 5.0 mM, pH = 7.0) from the related progress curves and Equation (4) as described in legend of Figure 1. as a function of activity (concentration) of MP-11, αo. Values of ki and αo were obtained (27°C, phosphate buffer 5.0 mM, pH = 7.0, [H2O2] = 1.0 mM) from the related progress curves and Equation (4) as described in the text and in the legend of Figure 1. c) αo as a function of concentration of hydrogen donor substrate, [AH]. Values of αo were obtained (27°C, phosphate buffer 5.0 mM, pH = 7.0, [H2O2] = 1.0 mM) from the related progress curves and Equation (4) as described in the text and in the legend of Figure 1.](/cms/asset/b5ea697e-610a-4171-9c64-4a23a583ffeb/ienz_a_226977_f0003_b.gif)
Figure 3 Linear plots based on the end-point procedure () illustrating dependence of initial concentration of AH ([AHi]) on the remaining (unreacted) concentration of AH ([AH∞]). Reactions were carried out at constant activity of MP-11. a) Linear plot based on Equation (5). A mixture of MP-11 (1.0 μM) and various initial concentrations of guaiacol ([AHi]) were incubated in the presence of [H2O2] = 1.0 mM at 27°C for 2 h (t = ∞). Reactions were carried out at constant activity of MP-11 (0.3 min− 1). Slope = Exp(–αi/ki) = 0.976 and αi/ki = 0.0243, Y-intercept = 0.009. b) Logarithmic plot based on Equation (6). A mixture of MP-11 (1.0 μM) and various initial concentrations of guaiacol ([AHi]) were incubated in the presence of [H2O2] = 1.0 mM at 27°C for 2 h (t = ∞). Reactions were carried out at constant activity of MP-11 (0.3 min− 1). Slope = 1.003, Y-intercept = –αi/ki = − 0.0256 and αi/ki = 0.0256 c): Linear plot according to Equation (7) for the variation of ln([AHi]/[AH∞]) with the initial activity of MP-11, αi. The mixture of AH and MP-11 (1.0 μM) were incubated in the presence of 1.0 mM H2O2, phosphate buffer 5.0 mM at 27°C for 2 h (t = ∞). Slope = 1/ki = 2.023 and ki = 0.494 min− 1., Y-intercept = 0.0.
![Figure 3 Linear plots based on the end-point procedure () illustrating dependence of initial concentration of AH ([AHi]) on the remaining (unreacted) concentration of AH ([AH∞]). Reactions were carried out at constant activity of MP-11. a) Linear plot based on Equation (5). A mixture of MP-11 (1.0 μM) and various initial concentrations of guaiacol ([AHi]) were incubated in the presence of [H2O2] = 1.0 mM at 27°C for 2 h (t = ∞). Reactions were carried out at constant activity of MP-11 (0.3 min− 1). Slope = Exp(–αi/ki) = 0.976 and αi/ki = 0.0243, Y-intercept = 0.009. b) Logarithmic plot based on Equation (6). A mixture of MP-11 (1.0 μM) and various initial concentrations of guaiacol ([AHi]) were incubated in the presence of [H2O2] = 1.0 mM at 27°C for 2 h (t = ∞). Reactions were carried out at constant activity of MP-11 (0.3 min− 1). Slope = 1.003, Y-intercept = –αi/ki = − 0.0256 and αi/ki = 0.0256 c): Linear plot according to Equation (7) for the variation of ln([AHi]/[AH∞]) with the initial activity of MP-11, αi. The mixture of AH and MP-11 (1.0 μM) were incubated in the presence of 1.0 mM H2O2, phosphate buffer 5.0 mM at 27°C for 2 h (t = ∞). Slope = 1/ki = 2.023 and ki = 0.494 min− 1., Y-intercept = 0.0.](/cms/asset/acd1b23f-6199-4a16-9e3d-07b827a6df48/ienz_a_226977_f0004_b.gif)
Figure 4 a) Dependence of ki on concentration of suicide-substrate, [H2O2]. [AH] = 0.4 mM, [MP-11] = 1.0 μM, pH = 7.0, phosphate buffer 5.0 mM and temperature of 27°C. b) Independency of αi on [H2O2]. [AH] = 0.4 mM, pH = 7.0, phosphate buffer 5.0 mM and temperature of 27°C.
![Figure 4 a) Dependence of ki on concentration of suicide-substrate, [H2O2]. [AH] = 0.4 mM, [MP-11] = 1.0 μM, pH = 7.0, phosphate buffer 5.0 mM and temperature of 27°C. b) Independency of αi on [H2O2]. [AH] = 0.4 mM, pH = 7.0, phosphate buffer 5.0 mM and temperature of 27°C.](/cms/asset/874b8e47-2c87-442c-b6f0-ed55e7ef3d9b/ienz_a_226977_f0005_b.gif)
Figure 5 Electronic spectra of native and inactivated microperoxidase 11 (150 μM) at pH = 7.0, phosphate buffer 5.0 mM. Solid line, Native MP-11 (in the absence of hydrogen peroxide). Dashed line, produced inactivated MP-11 (or P-670) after 5 min incubation with [H2O2] = 3.0 mM.
![Figure 5 Electronic spectra of native and inactivated microperoxidase 11 (150 μM) at pH = 7.0, phosphate buffer 5.0 mM. Solid line, Native MP-11 (in the absence of hydrogen peroxide). Dashed line, produced inactivated MP-11 (or P-670) after 5 min incubation with [H2O2] = 3.0 mM.](/cms/asset/4983bdf7-84cd-4a8f-8896-46fa1f780c2f/ienz_a_226977_f0006_b.gif)