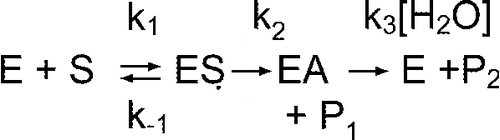Figures & data
Figure 2 Plot of velocity versus [S] for hydrolysis of o-NTFNAC by FAF-HSA ([E] = 0.075 mM) in 60 mM Tris/HCl buffer, pH 8.0 at 25°C. The continuous line is the fit to Equation (2). The velocity is expressed as the change in optical density (ΔOD) at λ = 430 nm per min.
![Figure 2 Plot of velocity versus [S] for hydrolysis of o-NTFNAC by FAF-HSA ([E] = 0.075 mM) in 60 mM Tris/HCl buffer, pH 8.0 at 25°C. The continuous line is the fit to Equation (2). The velocity is expressed as the change in optical density (ΔOD) at λ = 430 nm per min.](/cms/asset/ca8a0ee6-1fc2-4411-8da3-cc7cb4a3f75d/ienz_a_238282_f0003_b.gif)
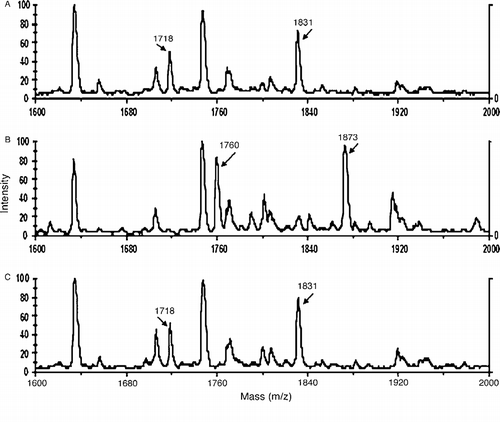
Table I. Expected sizes of Tyr 411 containing peptides after digestion of human albumin (accession# gi:28592) with pepsin, before and after reaction with substrates p-NPA or o-NTFNAC.
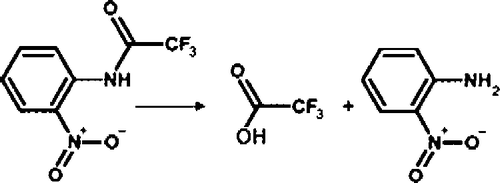
Figure 3 MALDI-TOF mass spectra showing the acyl-albumin adduct on Tyr 411. Panel A is untreated control albumin digested with pepsin. The Tyr 411 containing peptides at 1718 and 1831 amu are indicated. Panel B shows the acylated albumin peptides at 1760 and 1873 amu derived from the reaction of albumin with p-NPA. Panel C shows that the reaction of albumin with o-NTFNAC yields the same peptides as untreated control. The absence of acylated peptides for the reaction with o-NTFNAC suggests that deacylation is rapid with this substrate and that the rate determining step is the formation of the acyl-albumin adduct.