Figures & data
Figure 1 The hydrolysis of Tyr-Gly in striatum membrane preparations. (A) Time course of the contents of Tyr-Gly (○) and of its hydrolysis product Tyr (•) with 0.5 mg/mL protein. Each point represents the mean with S.E.M. of 5 independent experiments. (B) Protein concentration- dependence of the change in the contents of Tyr-Gly (▪) and Tyr (▪) in 2 h incubation period. Each column represents the mean with S.E.M. of 5 independent experiments. When the error bars are not shown, they are smaller than the symbol.
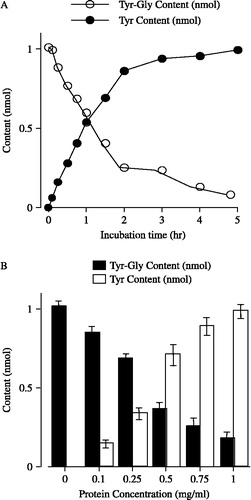
Figure 2 Effects of amastatin and AHPA-Val on the amounts of Tyr-Gly and its hydrolysis product Tyr in striatum membrane preparations, respectively. Each point represents the mean and vertical bar indicates the S.E.M. of 6 ∼ 8 preparations. For the “veh”, we incubated Tyr-Gly in Tris-HCl buffer without protein and APNIs. Vs. respective concentration of amastatin, * p < 0.05, *** p < 0.001 (unpaired t-test).
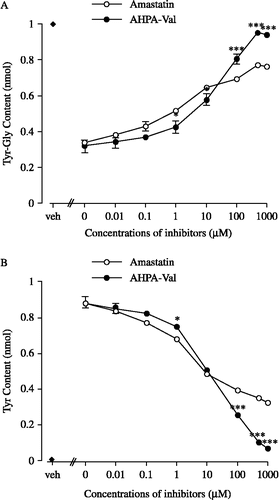
Figure 3 Kinetic analyses of amastatin (A) and AHPA-Val (B) on the hydrolysis of Tyr-Gly using Lineweaver-Burk plot of 1/V against 1/[S]. The inhibitory potencies of AHPA-Val and amastatin were measured using Tyr-Gly as substrate with the concentrations indicated (0.05 mM, 0.1 mM and 0.2 mM). Each point represents the mean of five separate experiments.
![Figure 3 Kinetic analyses of amastatin (A) and AHPA-Val (B) on the hydrolysis of Tyr-Gly using Lineweaver-Burk plot of 1/V against 1/[S]. The inhibitory potencies of AHPA-Val and amastatin were measured using Tyr-Gly as substrate with the concentrations indicated (0.05 mM, 0.1 mM and 0.2 mM). Each point represents the mean of five separate experiments.](/cms/asset/b1529047-e521-4a7b-ba57-3d4b2d88685a/ienz_a_251421_f0003_b.gif)
Figure 4 Effects of amastatin (A) and AHPA-Val (B) on Met-enkephalin-induced twitch inhibition in guinea pig ileum preparations. Peptidase inhibitors were applied accumulatively. Each point represents the mean percentage inhibition with S.E.M of 9 independent experiments.
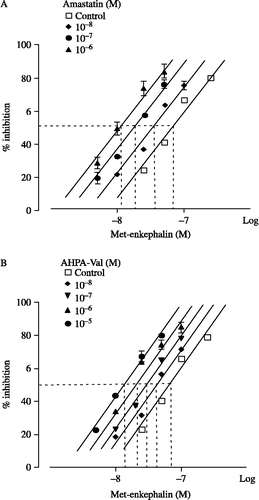
Table I. Effects of amastatin or AHPA-Val on the IC50 values of Met-enkephalin- induced twitch inhibition in guinea pig ileum preparations.
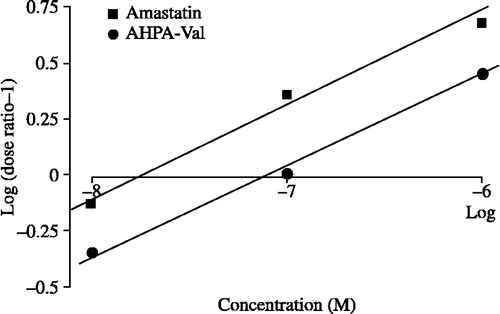
Figure 5 pA1/2 analyses of amastatin (▪) and AHPA-Val (•). pA1/2 value is defined as the negative log concentration of peptidase inhibitor that produced the effect to decrease the IC50 of Met-enkephalin in half. pA1/2 of amastatin and AHPA-Val is 7.79 and 7.08, respectively. Each point represents the mean of 9 independent experiments.